Selectively polymerizable compositions and methods of use in vivo
A composition and polymer technology, applied in the direction of drug combination, prosthesis, tissue regeneration, etc., can solve the problems of no application in dentistry, etc., achieve the effect of enhancing drug efficacy, reducing recurrent infection, and easy application
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
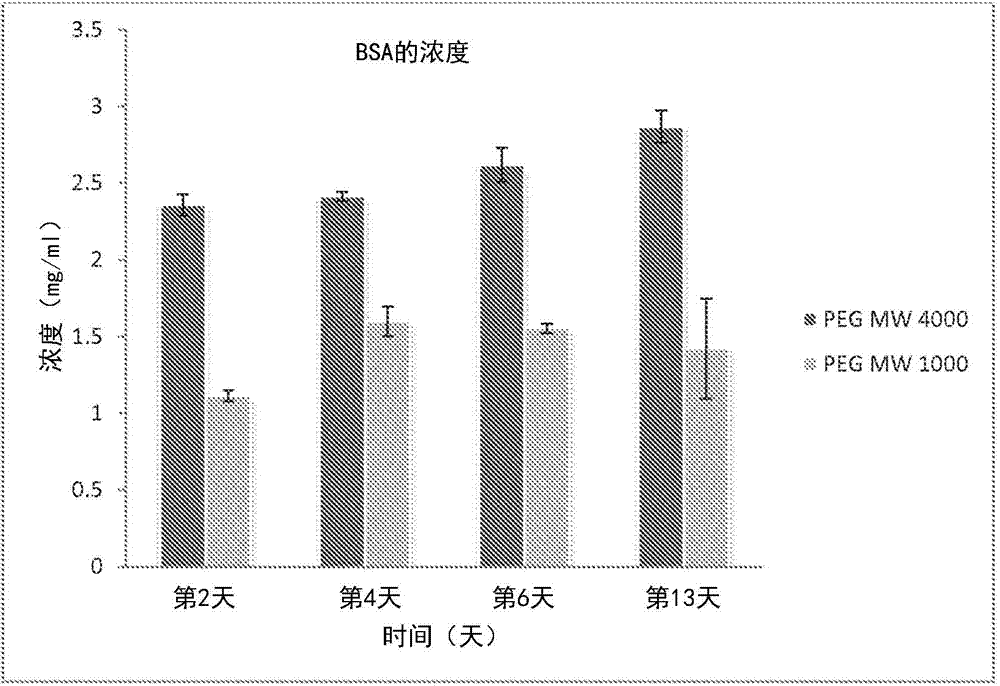
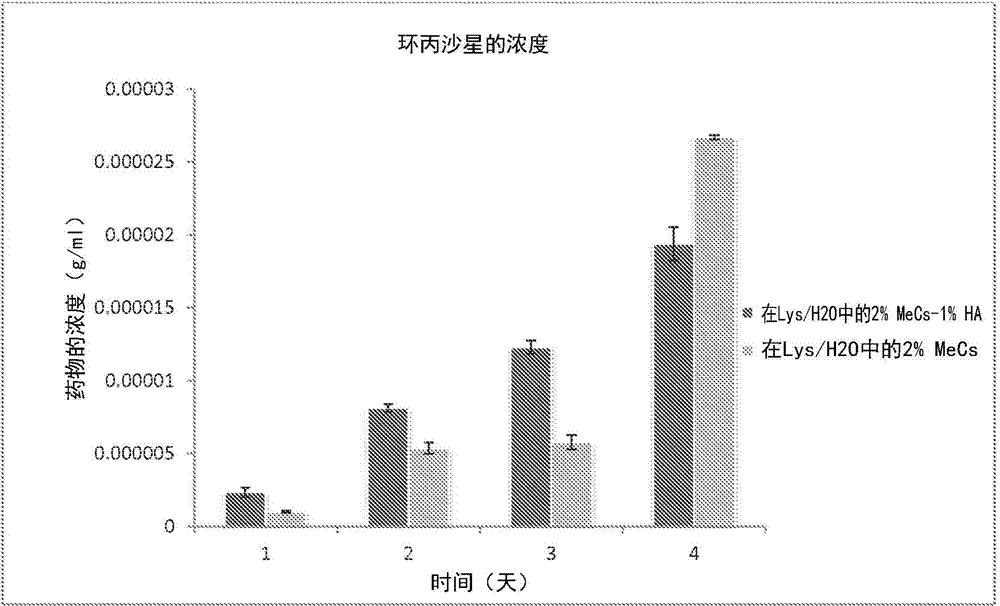
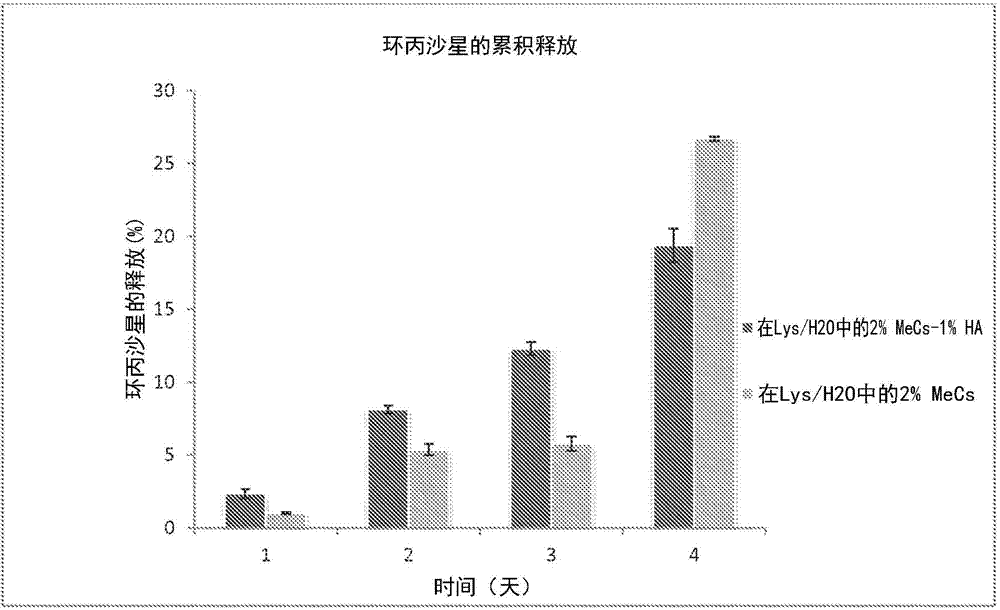
[0071]In laboratory tests, the precursor may include polyethylene glycol diacrylate having a molecular weight of 1000 or polyethylene glycol diacrylate having a molecular weight of 4000 purchased from Polysciences Inc. (Warrington, PA). Bovine serum albumin (BSA), tetramethylrhodamine conjugate can be purchased from Invitrogen Inc. (Eugene, OR).
[0072] Test drug incorporation and release. During the initial crosslinking process, BSA was loaded into the hydrogel. A pH 7.4, 10% w / v solution of BSA in PBS was prepared. A photocrosslinking solution of 10% w / v PEG in PBS containing 0.5% Irgacure 2959 photoinitiator was prepared. BSA was incorporated into the solution at 5 wt% relative to the macromer loading. The final solution was stirred at 350 rpm for 15 minutes. Pipette 200 µL into wells of a 96-well plate. Each construct was exposed to UV light for 55 seconds. The gel was immediately removed from the well using a spatula and placed in a separate vial containing 5ml of ...
Embodiment 2
[0074] In the clinic, one embodiment of the invention may include the following administrations. The patient can lie on their side with the ear under the microscope. This allows the doctor to view and manipulate the eardrum without taking up both hands. The physician at this point can administer the liquid prepolymer composition mixed with the desired medication. The composition can be instilled into the ear using a pipette, just as ear drops are administered. A doctor may only need tens of microliters of the composition to cover a piercing. Once the composition is added and covers the perforation, the physician can irradiate the composition with light where the hydrogel is desired. Although there is no need to withdraw the unused composition, excess composition can be removed by the physician at his preference using a pipette or some sort of absorbent. Now that all excess composition can be removed, any excess composition can be added to ensure that the perforation is cov...
Embodiment 3
[0076] In a surgical setting, one embodiment of the present invention may include optimizing surgical filling materials currently used for the middle ear. Before suturing the surgical wound, the liquid prepolymer composition already incorporated with the desired drug is dropped onto the filling material and polymerized with light. In addition to enhancing mechanical support, this embodiment would also impart certain antimicrobial and drug delivery properties not currently available to surgical filling materials, thereby enhancing postoperative outcomes.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com