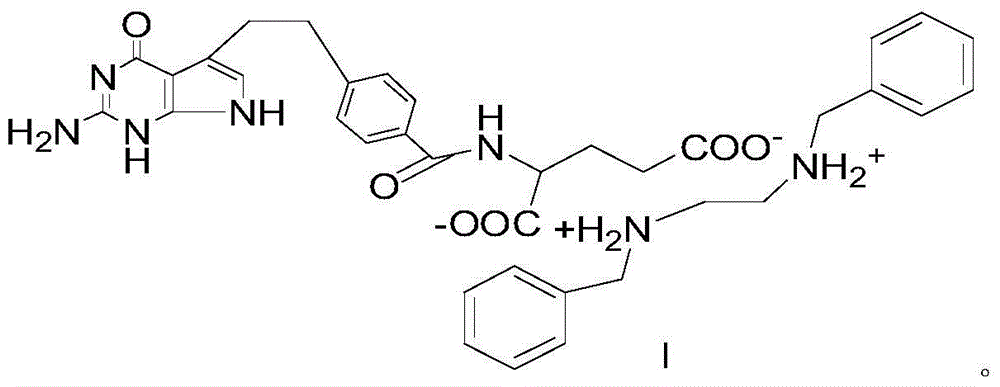
Pemetrexed-N,N-dibenzylethylenediamine salt and preparation method thereof
A technology of dibenzylethylenediamine salt and pemetrexed diacid is applied in the field of pemetrexed-N,N-dibenzylethylenediamine salt and preparation thereof, and can solve the problems of poor drug stability and preparation process. Complex problems, to achieve good stability, simple and reasonable steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
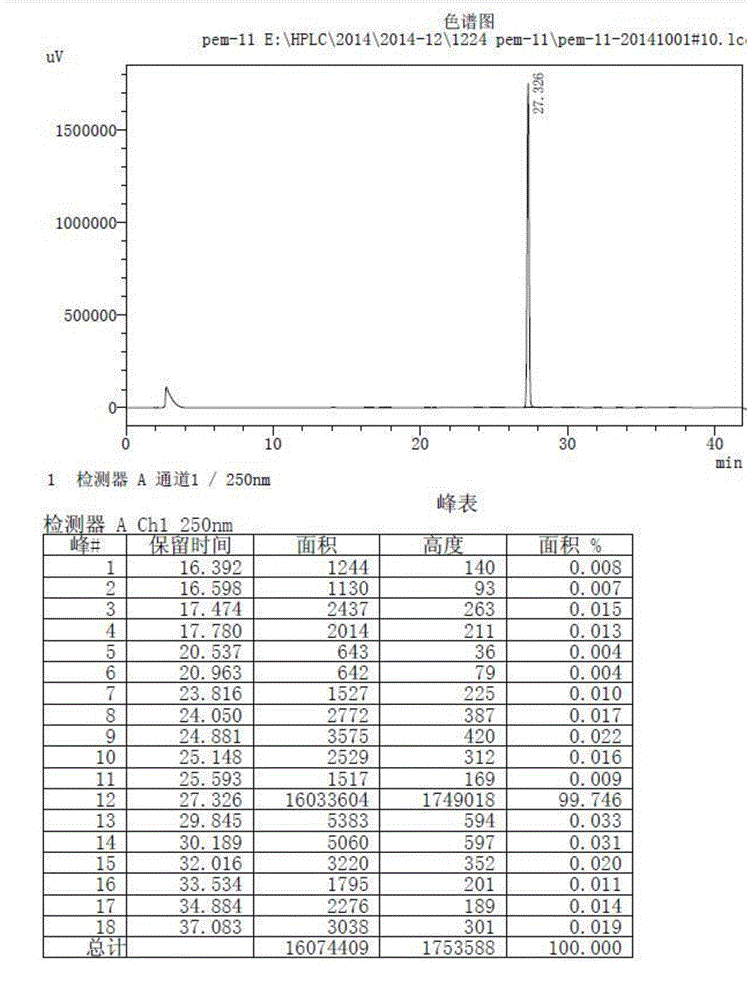
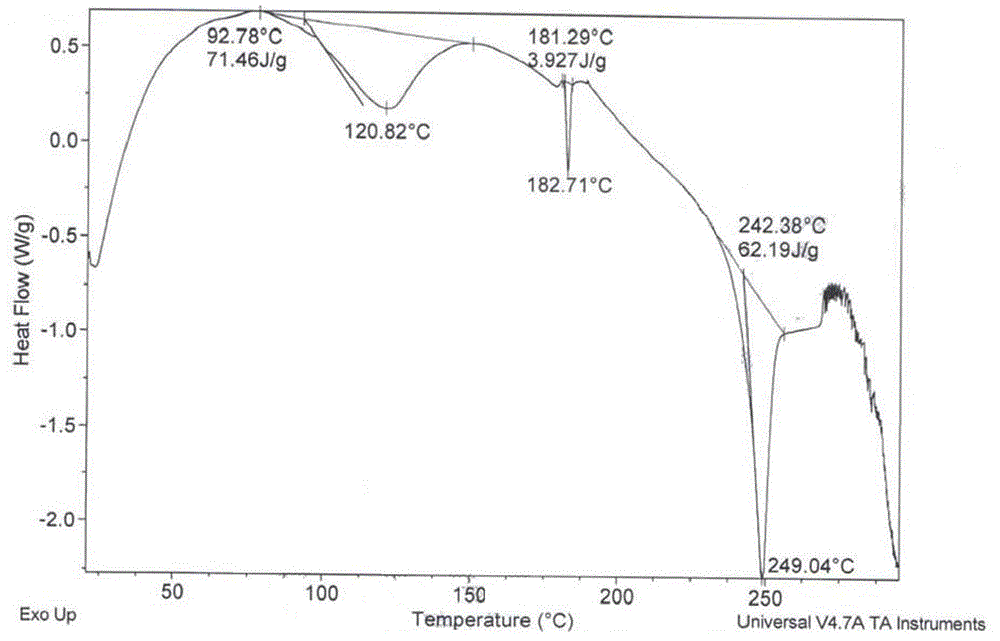
Image
Examples
Embodiment 1
[0031] In this embodiment, the preparation method of pemetrexed-N,N-dibenzylethylenediamine salt comprises the following steps:
[0032] (1)N 2 Under protection, put 5.0g of pemetrexed disodium heptahydrate into a 250mL four-neck flask filled with 100mL of purified water, and stir at 20°C until completely dissolved;
[0033] (2) in N 2 Under protection, add 26mL of acetic acid aqueous solution with a volume concentration of 23% to the system of step (1), and the aqueous acetic acid solution will be dripped within 30 minutes; the temperature of the control system is 20 ° C, and the reaction is stirred for 1 hour; Wash the filter cake with purified water and drain it twice, each time using 25 mL of purified water, then rinse the filter cake with absolute ethanol and drain it twice, each time using 10 mL of absolute ethanol; The filter cake was vacuum-dried at 40°C for 12 hours to obtain pemetrexed diacid;
[0034] (3) in N 2 Under protection, get the pemetrexed diacid that 3...
Embodiment 2
[0038] In this embodiment, the preparation method of pemetrexed-N,N-dibenzylethylenediamine salt comprises the following steps:
[0039] (1)N 2 Under protection, put 5.0g of pemetrexed disodium heptahydrate into a 250mL four-neck flask filled with 100mL of absolute ethanol, and stir at 35°C until completely dissolved;
[0040] (2) in N 2 Under protection, add 52mL of ethanol acetate solution with a volume concentration of 23% to the system of step (1), and the ethanol acetate solution will be dripped within 30min; the temperature of the control system is 35°C, and the reaction is stirred for 1.5h; Filter, rinse the filter cake with purified water and drain it twice, each time the amount of purified water is 25mL, then use absolute ethanol to rinse the filter cake and drain it twice, each time the amount of absolute ethanol is 10mL ; The obtained filter cake was vacuum-dried at 45° C. for 16 hours to obtain pemetrexed diacid;
[0041] (3) in N 2 Under protection, get the pe...
Embodiment 3
[0044]In this embodiment, the preparation method of pemetrexed-N,N-dibenzylethylenediamine salt comprises the following steps:
[0045] (1)N 2 Under protection, put 5.0 g of pemetrexed disodium heptahydrate into a four-necked flask filled with 100 mL of 50% ethanol aqueous solution, and stir at 25°C until completely dissolved;
[0046] (2) in N 2 Under protection, add 78mL of acetic acid aqueous solution with a volume concentration of 23% to the system of step (1), and the aqueous acetic acid solution will be dripped within 30min; the temperature of the control system is 25°C, and the reaction is stirred for 1.2h; after the reaction is completed, suction filtration, Rinse the filter cake with purified water and drain it twice, each time using 25 mL of purified water, then rinse the filter cake with absolute ethanol and drain it twice, each time using 10 mL of absolute ethanol; The resulting filter cake was vacuum-dried at 42°C for 14 hours to obtain pemetrexed diacid;
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com