A kind of di-n-butyltin o-phthaloacetate and its preparation method and application
A technology of di-n-butyltin o-phthalic acid ester and o-phthalic acid ester is applied in the application field of di-n-butyl tin o-phthalic acid ester in plant growth regulation, which can solve the problem of producing drug resistance and the like. problem, to achieve the effect of low cost, simple preparation method and high herbicidal activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Preparation of di-n-butyltin phthalate:
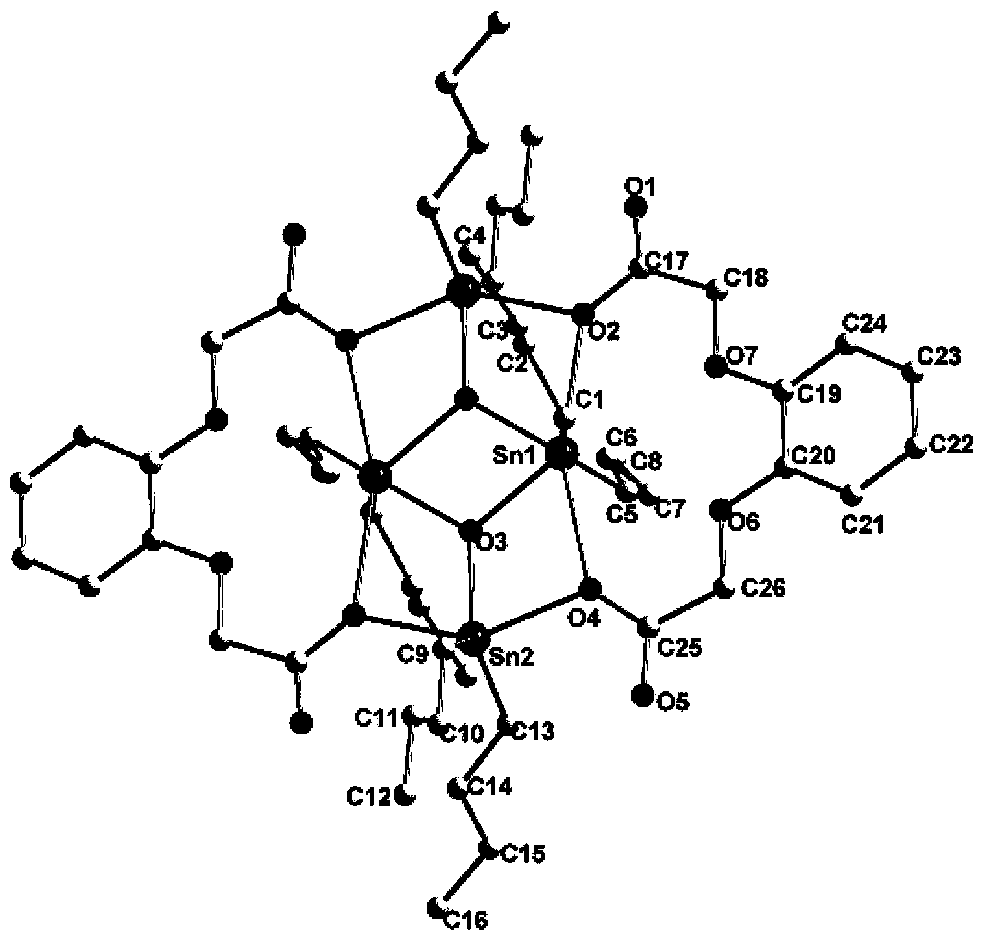
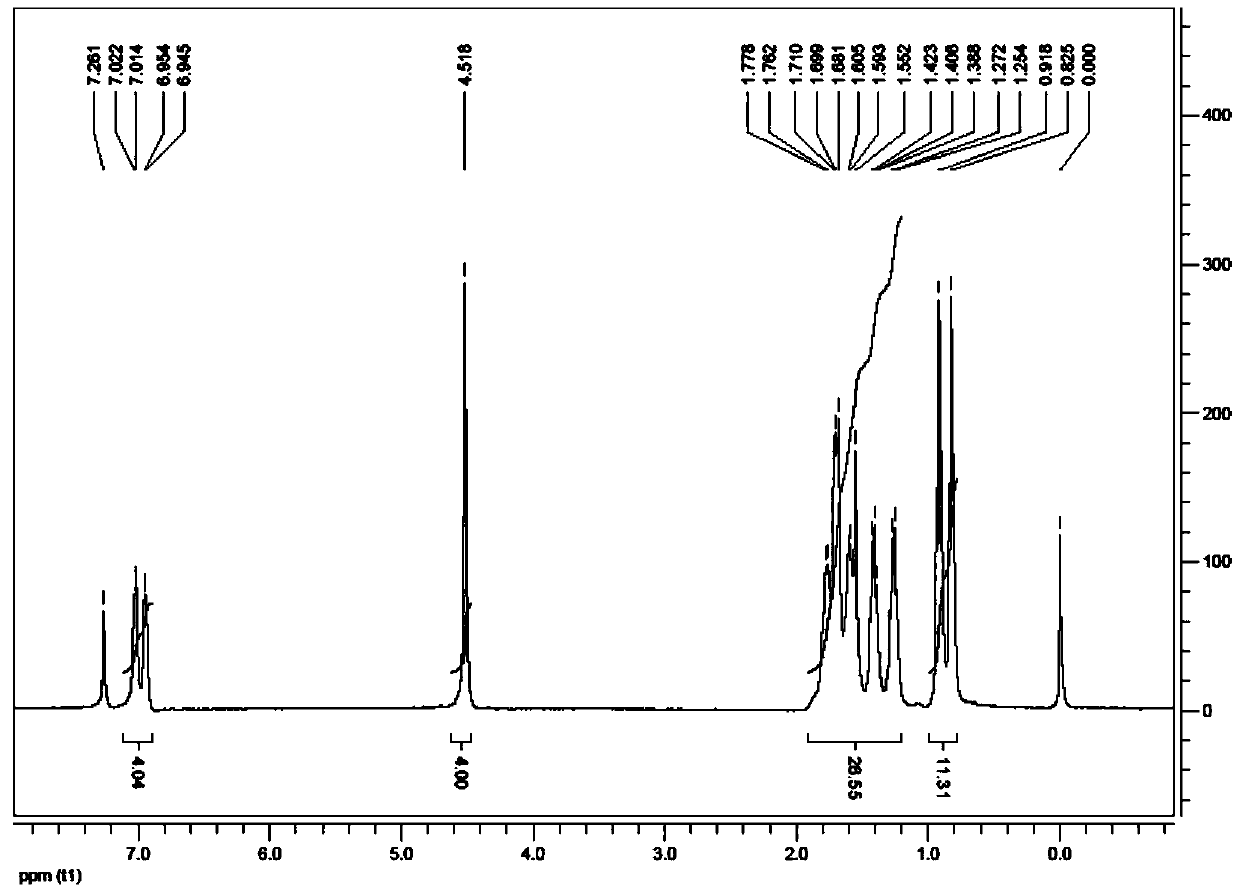
[0039] Add 0.226g (1.0mmol) phthalic acid, 0.496g (2.0mmol) dibutyltin oxide, and 25ml anhydrous methanol to a 50mL three-necked flask protected by nitrogen, stir and reflux for 5h, after the reaction is complete, cool to room temperature , filtered, and at a pressure of 0.005MPa and a temperature of 30°C, the filtrate was evaporated to dryness with a rotary evaporator to obtain a white solid, which was recrystallized with a dichloromethane-methanol mixed solvent, wherein the volume ratio of dichloromethane to methanol was 1: 10. Under the condition of 15-35°C, control solvent volatilization and crystallization to obtain colorless transparent crystals, which are di-n-butyltin phthalate of the present invention. Yield: 58.7%. Melting point: 280℃~282℃.
[0040] Elemental analysis (C 52 h 88 o 14 sn 4 ): Theoretical: C 44.23, H 6.28; Found: C 44.25, H 6.27.
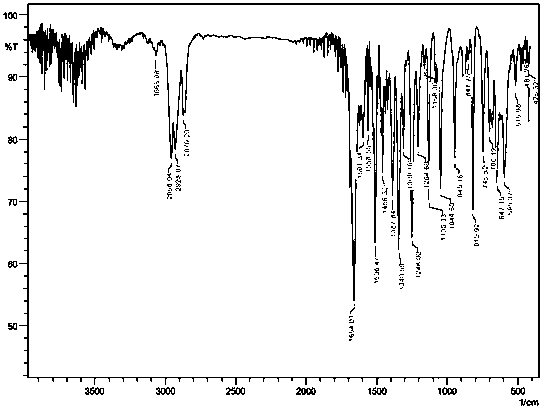
[0041] IR(KBr,cm -1): 2956(s), 2928(s), 1654(vs), 1506(s), 1340(s...
Embodiment 2
[0046] Preparation of di-n-butyltin phthalate:
[0047] Add 0.226g (1.0mmol) of phthalic acid, 0.570g (2.3mmol) of dibutyltin oxide, and 25ml of absolute ethanol into a 50mL three-necked flask protected by nitrogen, stir and reflux for 7h, after the reaction is completed, cool to room temperature , filtered, and at a pressure of 0.007MPa and a temperature of 40°C, the filtrate was evaporated to dryness with a rotary evaporator to obtain a white solid, which was recrystallized with a dichloromethane-methanol mixed solvent, wherein the volume ratio of dichloromethane to methanol was 1: 15. Under the condition of 15-35°C, control solvent volatilization and crystallization to obtain colorless transparent crystals, which are di-n-butyltin phthalate of the present invention. Yield: 58.9%. Melting point: 280℃~282℃.
[0048] Elemental analysis (C 52 h 88 o 14 sn 4 ): Theoretical: C 44.23, H 6.28; Found: C 44.25, H 6.27.
[0049] IR(KBr,cm -1 ): 2956(s), 2928(s), 1654(vs), 1506...
Embodiment 3
[0054] Preparation of di-n-butyltin phthalate:
[0055] Add 1.130g (5.0mmol) of phthalic acid, 2.728g (11.0mmol) of dibutyltin oxide, and 70ml of anhydrous benzene into a 150mL three-necked flask protected by nitrogen, stir and reflux for 10h. After the reaction is completed, cool to room temperature , filtered, and at a pressure of 0.006MPa and a temperature of 38°C, the filtrate was evaporated to dryness with a rotary evaporator to obtain a white solid, which was recrystallized with a dichloromethane-methanol mixed solvent, wherein the volume ratio of dichloromethane to methanol was 1: 6. Under the condition of 15-35°C, control solvent volatilization and crystallization to obtain colorless transparent crystals, which are the di-n-butyltin phthalate of the present invention. Yield: 57.6%. Melting point: 280℃~282℃.
[0056] Elemental analysis (C 52 h 88 o 14 sn 4 ): Theoretical: C 44.23, H 6.28; Found: C 44.25, H 6.27.
[0057] IR(KBr,cm -1 ): 2956(s), 2928(s), 1654(vs...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com