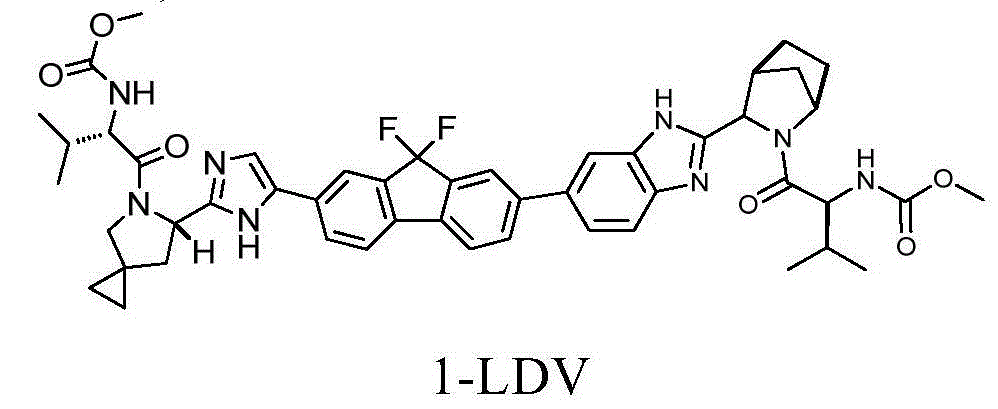
Preparation method of ledipasvir and derivative thereof, and intermediate compound for preparing the ledipasvir
A technology of compound and coupling reaction, which is applied in the preparation of the compound of formula 1, the preparation of intermediate compounds of ledipasvir, the preparation of ledipasvir and its derivatives, and can solve the problem of low yield and increased discharge of three wastes, etc. question
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
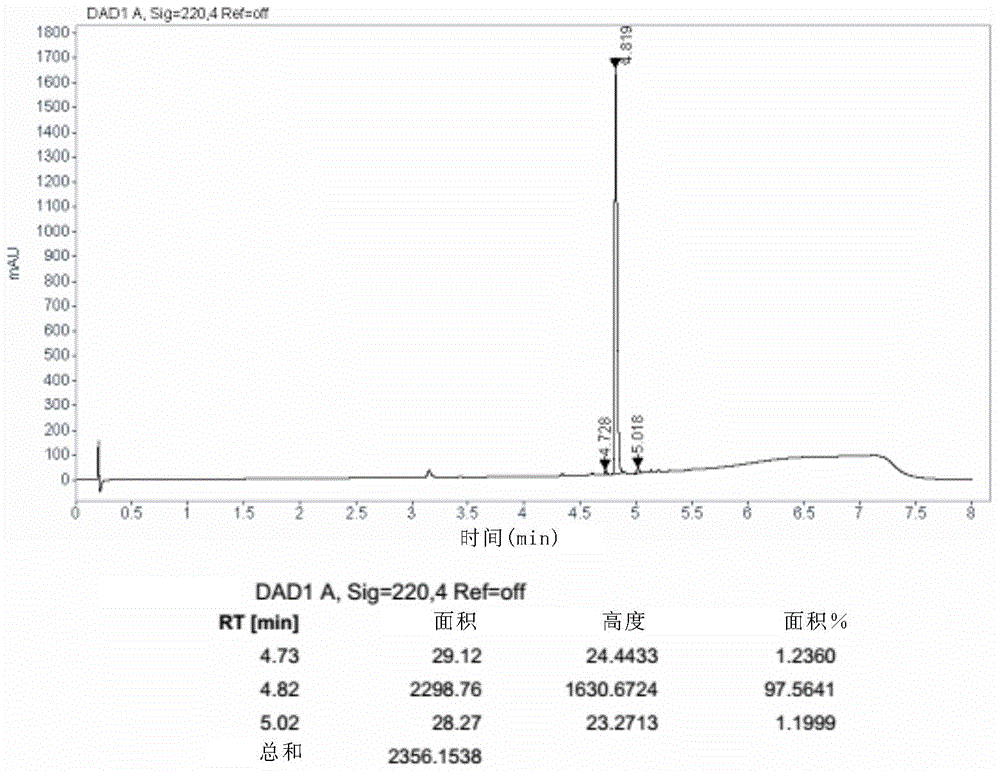
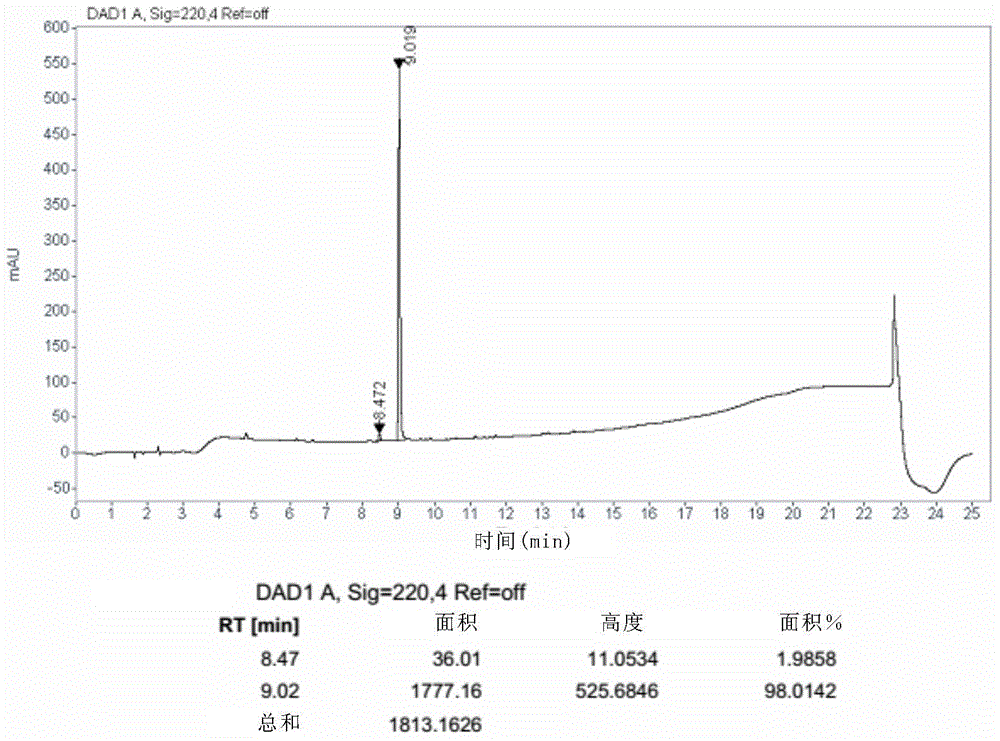
Image
Examples
preparation example Construction
[0160] The present invention provides various preparation methods of ledipasvir, and the preparation methods include one or more of the following features:
[0161] 1) The reaction temperature is 0-100°C, preferably 20-90°C;
[0162] 2) The reaction time is 0.1-48h, preferably 2-20h;
[0163] 3) The reaction is carried out in an inert solvent.
[0164] The preferred preparation method of the present invention is shown in Scheme 1-3.
[0165] Route 1
[0166]
[0167] In this method, the Moc-val group is firstly introduced into compound 11 without Boc protection, which can significantly improve the synthesis efficiency and reduce the emission of three wastes.
[0168] route 2
[0169]
[0170] In this method, compound 11 and compound 3-Moc are first introduced into Moc-Val, which eliminates the protection and deprotection reactions, greatly reduces the synthesis steps, improves the synthesis efficiency, significantly reduces the production cycle, significantly reduces...
Embodiment 1
[0178] Embodiment 1: the synthesis of compound 12-Br-Cbz
[0179]
[0180] Add compound 10-Br-Cl (2.03g, 5.675mmol), compound 21 (1.72g, 6.243mmol), DIPEA (0.81g, 6.243mmol) and acetonitrile (40mL) in the there-necked flask, heat to 70°C, stir for 5 hour, then cooled to room temperature, distilled off the solvent, then added ethyl acetate (100mL), washed once with dilute hydrochloric acid (0.01M / L, 200mL), dried the organic phase with anhydrous sodium sulfate, and distilled off the solvent to obtain the product ( 3.384 g, yield 100%).
Embodiment 2
[0181]Embodiment 2: the synthesis of compound 2-Br-Cbz
[0182]
[0183] Add compound 12-Br-Cbz (3.384g, 5.675mmol), ammonium acetate (2.187g, 28.375mmol), ethylene glycol monomethyl ether (4mL) and toluene (70mL) in a three-necked flask, heat to 90°C, stir 5 hours, then cooled to room temperature, then added ethyl acetate (100mL), washed twice with brine (200mL), the organic phase was dried over anhydrous sodium sulfate, and the product (3.2g, yield 98% ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com