Synthesis process of ledipasvir intermediate
A synthesis process and intermediate technology, applied in the field of synthesis process of ledipasvir intermediates, can solve anemia, allergies and depression-like psychosis, high difficulty in separation of catalyst and reaction liquid, increased cost of industrial waste liquid treatment, etc. problems, to achieve the effect of convenient separation, high yield and increased specific surface area
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] A synthetic technique for a ledipasvir intermediate, specifically comprising the steps of:
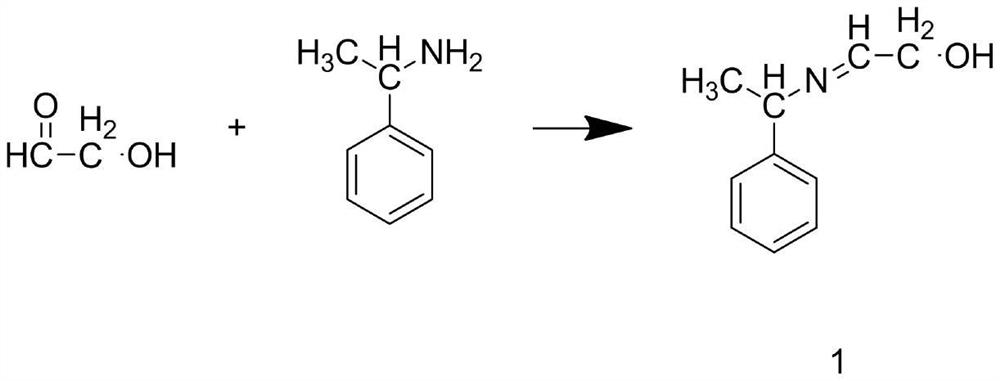
[0044] Step S1: add glycolaldehyde into deionized water, stir until glycolaldehyde is completely dissolved, and prepare glycolaldehyde solution, add α-phenylethylamine and dichloromethane into the reaction kettle, at a speed of 200r / min, At a temperature of 25°C, stir until α-phenylethylamine is completely dissolved, continue stirring and add glycolaldehyde solution and anhydrous sodium sulfate, and react for 5 hours to obtain Intermediate 1;
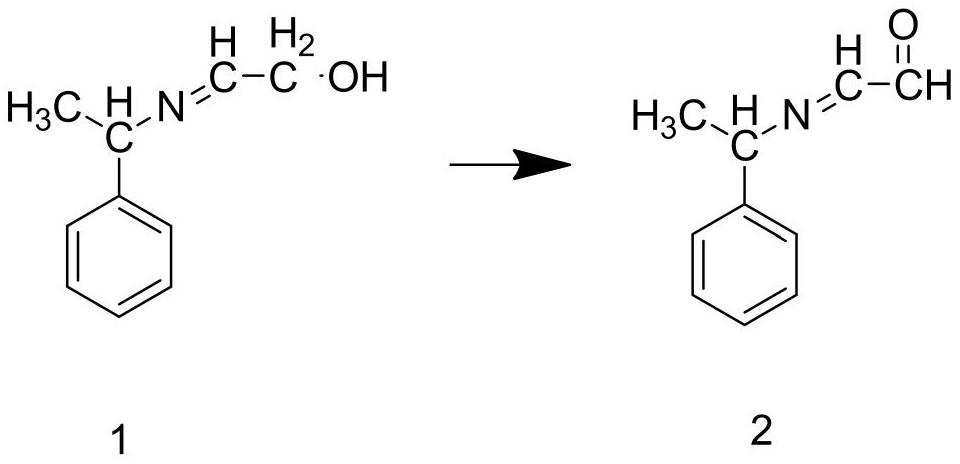
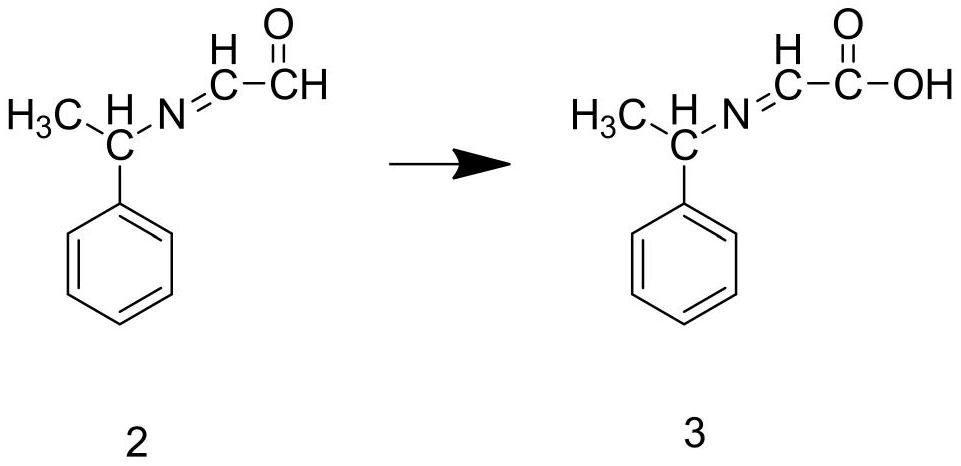
[0045]Step S2: Add the intermediate 1 and deionized water prepared in step S1 into the reaction kettle, stir until the intermediate 1 is completely dissolved, add copper powder, and react for 1.5 hours at a temperature of 80°C to prepare To obtain intermediate 2, add intermediate 2 and acidic potassium permanganate solution into the reaction kettle, and react for 3 hours at a temperature of 50°C to obtain intermediate 3;
[0046] Step S3...
Embodiment 2
[0054] A synthetic technique for a ledipasvir intermediate, specifically comprising the steps of:
[0055] Step S1: add glycolaldehyde into deionized water, stir until glycolaldehyde is completely dissolved, and prepare glycolaldehyde solution, add α-phenylethylamine and dichloromethane into the reaction kettle, at a speed of 220r / min, At a temperature of 26°C, stir until α-phenylethylamine is completely dissolved, continue stirring and add glycolaldehyde solution and anhydrous sodium sulfate, and react for 4.8 hours to obtain Intermediate 1;
[0056] Step S2: Add the intermediate 1 and deionized water prepared in step S1 into the reaction kettle, stir until the intermediate 1 is completely dissolved, add copper powder, and react for 1.3 hours at a temperature of 82°C. To obtain intermediate 2, add intermediate 2 and acidic potassium permanganate solution into the reaction kettle, and react at a temperature of 53°C for 2.8 hours to obtain intermediate 3;
[0057] Step S3: Met...
Embodiment 3
[0065] A synthetic technique for a ledipasvir intermediate, specifically comprising the steps of:
[0066] Step S1: add glycolaldehyde into deionized water, stir until glycolaldehyde is completely dissolved, and prepare glycolaldehyde solution, add α-phenylethylamine and dichloromethane into the reaction kettle, at a speed of 260r / min, At a temperature of 28°C, stir until α-phenylethylamine is completely dissolved, continue to stir, add glycolaldehyde solution and anhydrous sodium sulfate, and react for 4.5 hours to obtain Intermediate 1;
[0067] Step S2: Add the intermediate 1 and deionized water prepared in step S1 into the reaction kettle, stir until the intermediate 1 is completely dissolved, add copper powder, and react for 1.4 hours at a temperature of 88°C. To obtain intermediate 2, add intermediate 2 and acidic potassium permanganate solution into the reaction kettle, and react at a temperature of 57°C for 2.5 hours to obtain intermediate 3;
[0068] Step S3: Methano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com