Method for the electrochemical production of 2,2,4-trimethyl adipic acid and 2,4,4-trimethyl adipic acid
A technology of trimethyl adipic acid and trimethyl cyclohexanol, applied in electrodes, electrolysis process, electrolysis components, etc., can solve problems such as corrosion safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
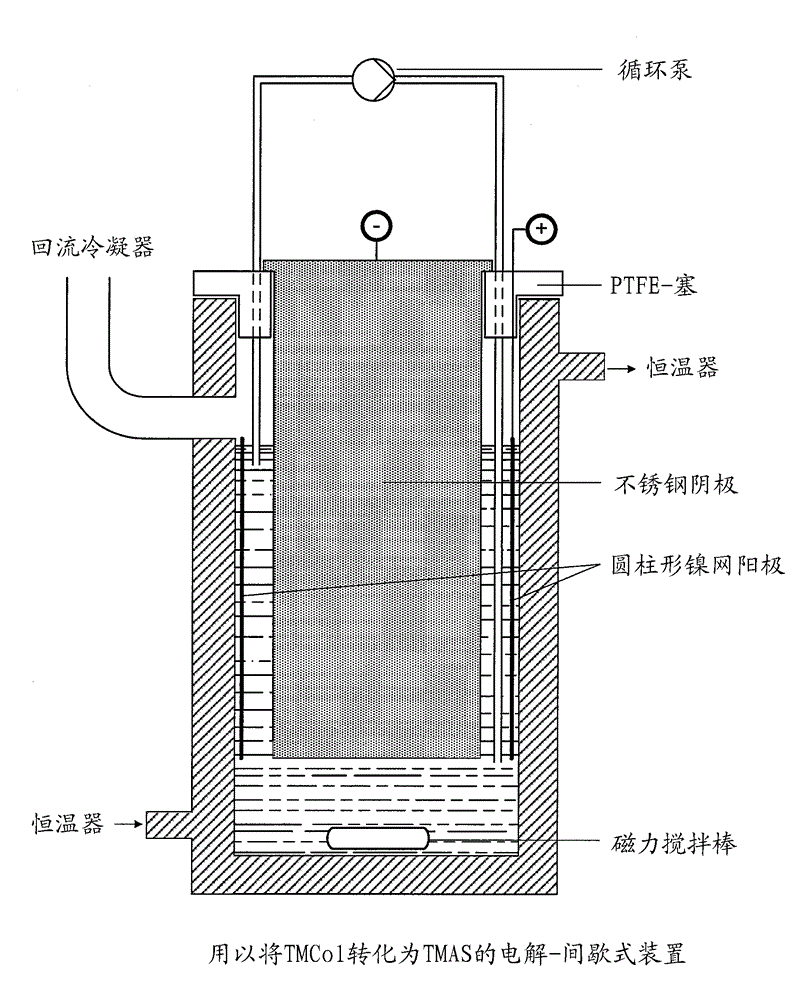
[0042] exist figure 1 The electrolysis was carried out as described above in the batch electrolysis apparatus shown in , having a coiled 100-mesh nickel mesh with an area of 10 cm*25 cm made of 0.1 mm nickel wire and a Round stainless steel plunger with a diameter of 7 cm.
[0043] 260 ml of water, 11.2 g of sodium hydroxide and 5.73 g of TMCol were injected into the electrolytic cell.
[0044] Electrolysis was carried out by introducing a current of 2 A through the electrolytic cell for 6 hours. The temperature is 80°C.
[0045] After work-up, 2.45 g TMCol, 2.34 g TMCon and 1.06 g TMAS were isolated. The yield of TMAS was 14%, based on the TMCol used.
Embodiment 2
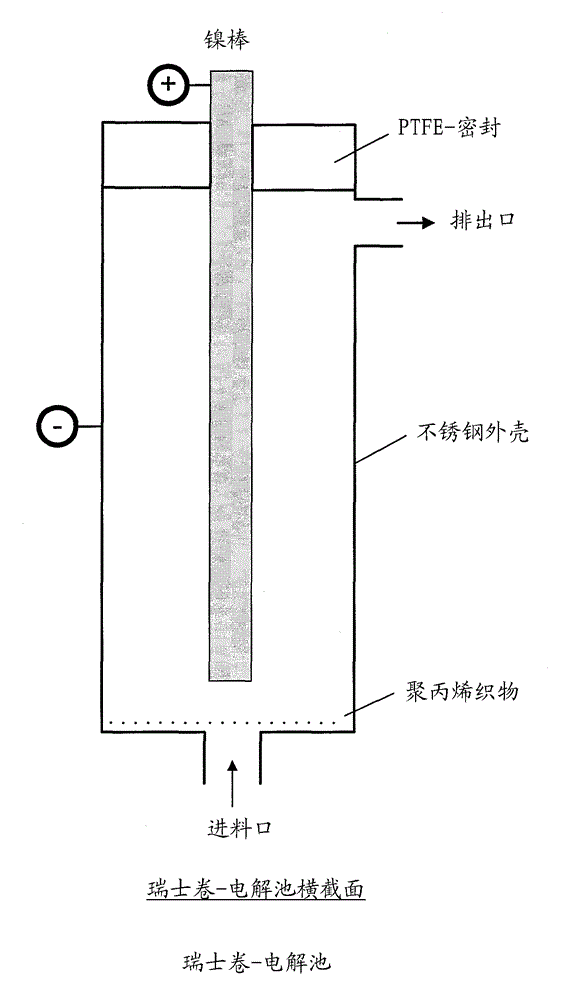
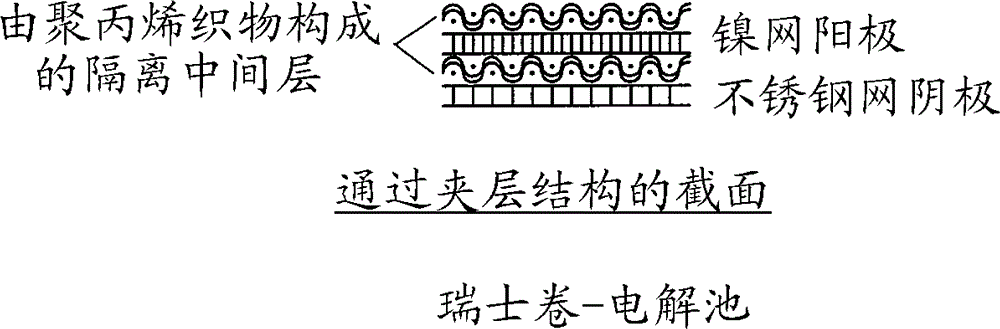
[0047] exist figure 2 The electrolysis was performed as described above using the Swiss-coil electrolytic cell shown in Figure 3 with a coiled area of 6.5 cm*24.5 cm made of 0.1 mm nickel wire in the electrolysis apparatus shown in FIG. 100-mesh nickel mesh, polypropylene fabric, and a 100-mesh stainless steel mesh with a coiled area of 6.5 cm*26.5 cm made of 0.114 mm stainless steel wire.
[0048] 260 ml of water, 11.2 g of sodium hydroxide and 5.93 g of TMCol were injected into the electrolysis unit. Use a peripheral pump.
[0049] Electrolysis was carried out by introducing a current of 2 A through the electrolytic cell for 24 hours. The temperature is 80°C.
[0050] After work-up, 0.05 g TMCol, 0.03 g TMCon and 2.70 g TMAS were isolated. The yield of TMAS was 34%, based on the TMCol used.
Embodiment 3
[0052] exist figure 2 used in the electrolysis device shown in Figure 4a and 4b with nickel particles (random volume 60 cm 3 ) electrolytic cell for electrolysis as described above.
[0053] 264 ml of water, 11.2 g of sodium hydroxide and 5.93 g of TMCol were injected into the electrolysis unit. Use a peripheral pump.
[0054] Electrolysis was carried out by introducing a current of 2 A through the electrolytic cell for 24 hours. The temperature is 80°C.
[0055] After work-up, 0.06 g TMCol, 0.04 g TMCon and 2.52 g TMAS were isolated. The yield of TMAS was 32%, based on the TMCol used.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com