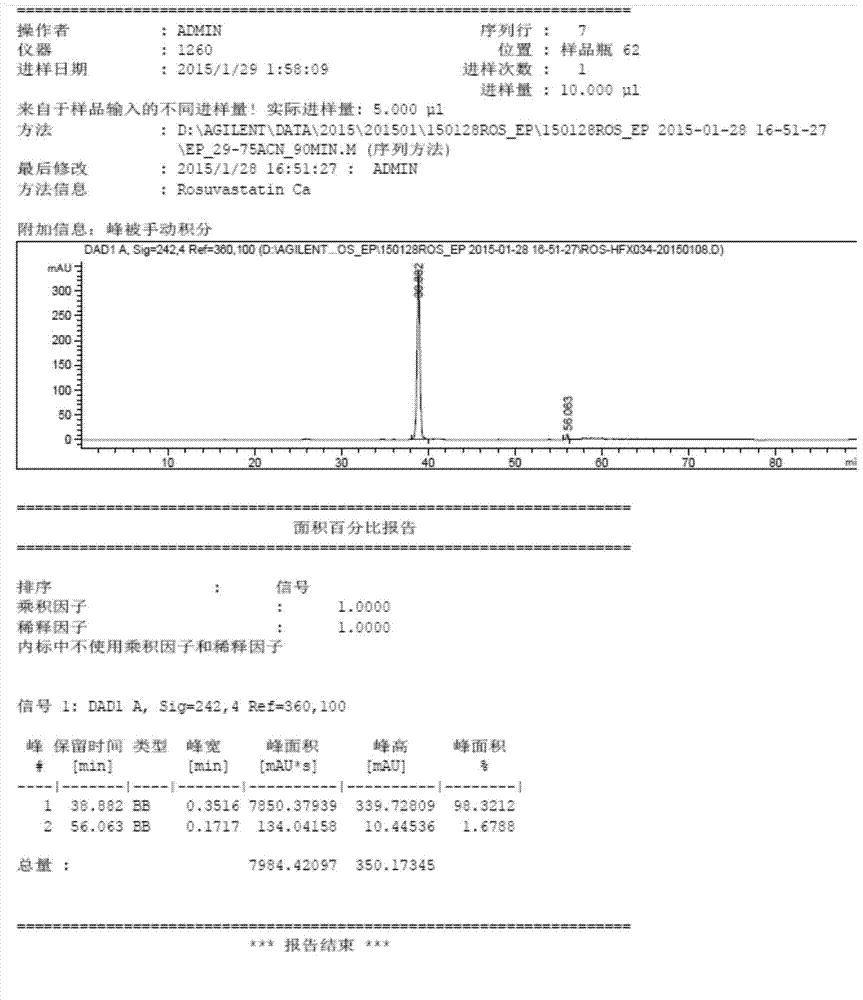
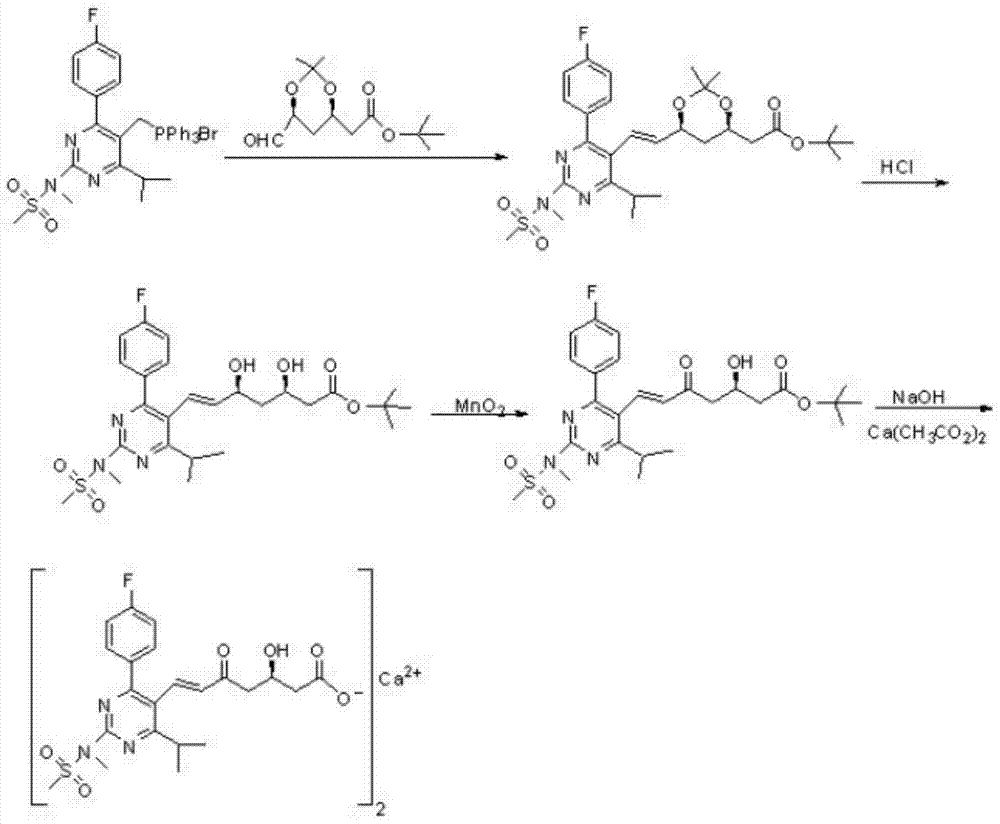
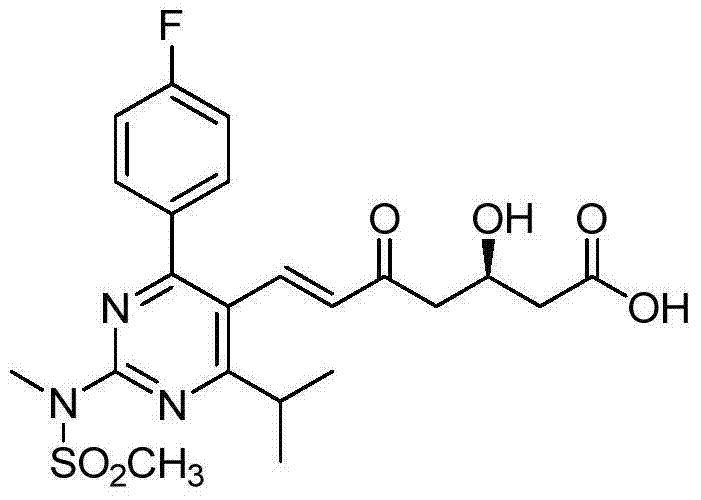
Preparation method of rosuvastatin calcium impurity
A technology for rosuvastatin calcium and impurities, which is applied in the field of drug synthesis, can solve the problems of limiting the overall yield, complex synthesis process, low substrate yield and the like, and achieves high product purity, high yield and convenient post-processing. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Compound 7-[4-(4-fluorophenyl)-6-isopropyl-2(N-methyl-N-methanesulfonamido)pyrimidin-5-yl]-(3R)-hydroxyl-5 -The preparation method of carbonyl-6-(E)-heptenoic acid adopts the following steps:
[0035] A. Compound 7-[4-(4-fluorophenyl)-6-isopropyl-2(N-methyl-N-methanesulfonamido)pyrimidin-5-yl]-(3R)-3-tert Preparation of butyldimethylsilyloxy-5-carbonyl-6-(E)-heptenoic acid methyl ester (H1):
[0036] In a 500ml four-necked flask, add 4-(4-fluorophenyl)-6-isopropyl-2(N-methyl-N-methanesulfonamido)pyrimidin-5-yl]-formaldehyde (Z9, 10g, 28.5 mmol), (3R)-3-tert-butyldimethylsilyloxy-5-carbonyl-6-(triphenylphosphinovinyl)-hexanoic acid methyl ester (J6, 16.76 g, 31.3 mmol) and acetonitrile 250ml, mechanical stirring, reflux reaction for about 30h, HPLC monitoring, after the reaction is complete, concentrate under reduced pressure to dryness, add 60ml of cyclohexane and rotary evaporate twice, then add 200ml of cyclohexane, reflux for 1h, cool to Incubate at 10-15°C for 2 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com