A class of substituted homopiperazine aminocarbamate bismuth complexes, pharmaceutically acceptable salts of the complexes, preparation methods and anti-tumor applications of the complexes
A technology of bismuth aminocarbamate and homopiperazine, which is applied in the field of antitumor drugs, can solve the problems of few antitumor cell lines, low clinical application value, and single structure, and achieve significant antitumor activity, good antitumor effect, and widespread The effect of applying the foreground
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
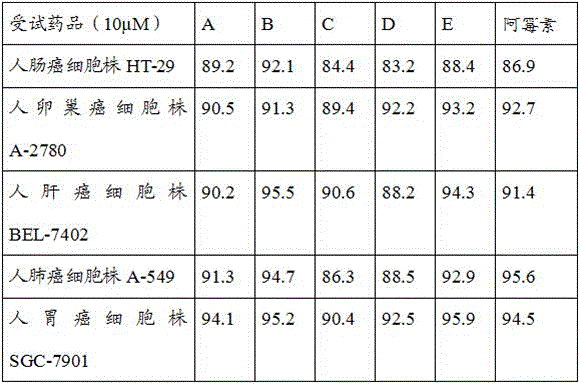
[0022] The present invention will be further described below through specific examples, but not as a limitation.
[0023] A, B, C, D, and E in the examples are used as numbers.
[0024] Implementation 1: Preparation of 4-methylhomopiperazine bismuth dicarbamate (A)
[0025] Add 0.571 g (5 mmol) of 1-methylhomopiperazine, 0.25 g (6 mmol) of sodium hydroxide, and 10-15 mL of anhydrous methanol into a 50 mL round bottom flask, and stir magnetically in a cold water bath at 0-5 °C After dissolving, add dropwise 0.76g (10 mmol) CS 2 , low temperature magnetic stirring reaction for 1 h, 10-50 ° C magnetic stirring reaction for 3 h.
[0026] 0.53 g (1.7 mmol) BiCl 3 Dissolve it in 5-15 mL of anhydrous methanol, drop it into the above reaction solution, add a certain amount of dichloromethane, and stir at room temperature for 3 h. After the reaction, a certain amount of water was added, the dichloromethane layer was separated, washed with water, dried over anhydrous sodium sulfate,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com