Chemical ice pack and preparation method thereof
An ice pack and chemical technology, applied in the field of chemistry, can solve the problems of short cooling time, rapid changes, unfavorable long-term cooling effect, etc., and achieve the effect of increasing cooling time and increasing cooling time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
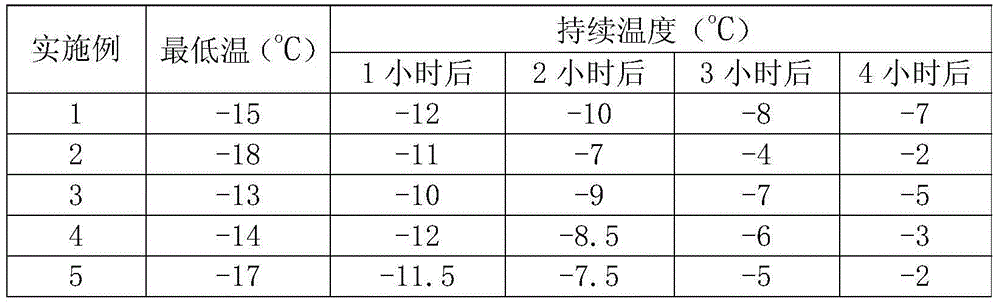
Embodiment 1
[0018] A chemical ice pack made of the following components by weight: 45 parts of ammonium nitrate, 75 parts of potassium aluminum sulfate dodecahydrate, 4 parts of sodium carboxymethylcellulose, 6 parts of ethanol, and 23 parts of sodium chloride , 3 parts of sodium hydroxide.
[0019] The preparation method of described chemical ice pack, comprises the steps:
[0020] (1) Accurately weigh according to the weight ratio of each component, add the weighed sodium carboxymethylcellulose into half potassium aluminum sulfate dodecahydrate and stir at a stirring speed of 250r / min, and heat to 85°C, When the solution is transparent, stop heating and allow it to cool down to room temperature naturally to obtain solution A;
[0021] (2) Add ammonium nitrate, the other half of potassium aluminum sulfate dodecahydrate, and ethanol into solution A in turn under stirring, and stir it evenly to obtain solution B;
[0022] (3) In solution B, first add sodium hydroxide and stir evenly, the...
Embodiment 2
[0024] A chemical ice pack made of the following components by weight: 40 parts of ammonium nitrate, 70 parts of potassium aluminum sulfate dodecahydrate, 2 parts of sodium carboxymethylcellulose, 9 parts of ethanol, and 30 parts of sodium chloride , 4 parts of sodium hydroxide.
[0025] The preparation method of described chemical ice pack, comprises the steps:
[0026] (1) Accurately weigh according to the weight ratio of each component, add the weighed sodium carboxymethyl cellulose into half potassium aluminum sulfate dodecahydrate and stir at a stirring speed of 200r / min, and heat to 80°C, When the solution is transparent, stop heating and allow it to cool down to room temperature naturally to obtain solution A;
[0027] (2) Add ammonium nitrate, the other half of potassium aluminum sulfate dodecahydrate, and ethanol into solution A in turn under stirring, and stir it evenly to obtain solution B;
[0028] (3) In solution B, first add sodium hydroxide and stir evenly, th...
Embodiment 3
[0030] A chemical ice pack, made of the following components by weight: 50 parts of ammonium nitrate, 80 parts of potassium aluminum sulfate dodecahydrate, 5 parts of sodium carboxymethylcellulose, 4 parts of ethanol, and 15 parts of sodium chloride , 1 part of sodium hydroxide.
[0031] The preparation method of described chemical ice pack, comprises the steps:
[0032] (1) Accurately weigh according to the weight ratio of each component, add the weighed sodium carboxymethyl cellulose into half potassium aluminum sulfate dodecahydrate and stir at a stirring speed of 300r / min, and heat to 90°C, When the solution is transparent, stop heating and allow it to cool down to room temperature naturally to obtain solution A;
[0033] (2) Add ammonium nitrate, the other half of potassium aluminum sulfate dodecahydrate, and ethanol into solution A in turn under stirring, and stir it evenly to obtain solution B;
[0034] (3) In solution B, first add sodium hydroxide and stir evenly, th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com