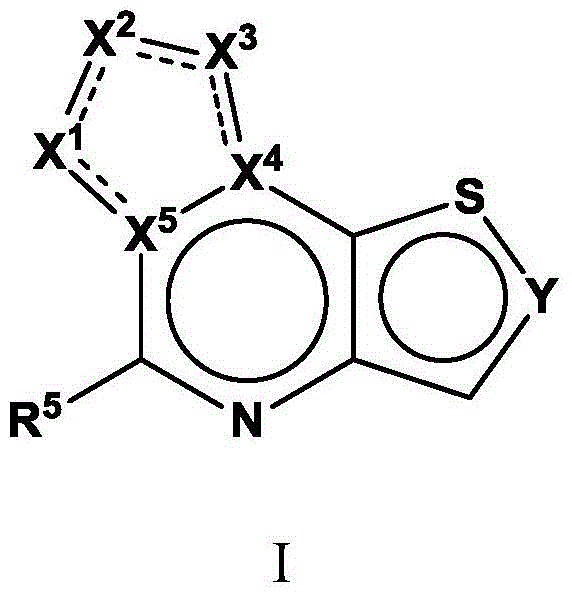
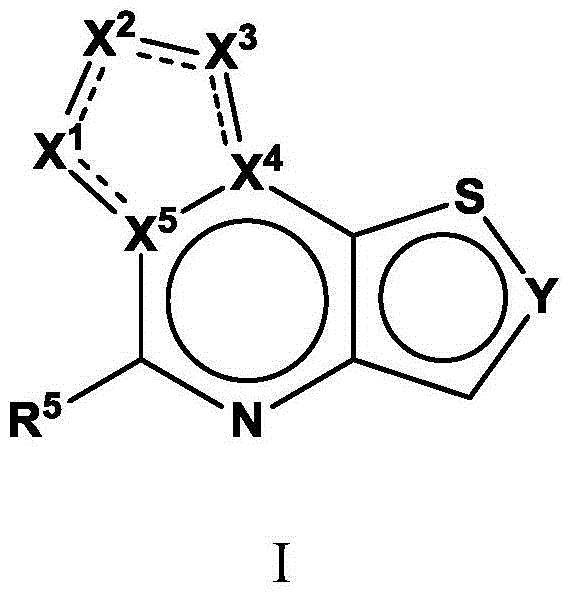
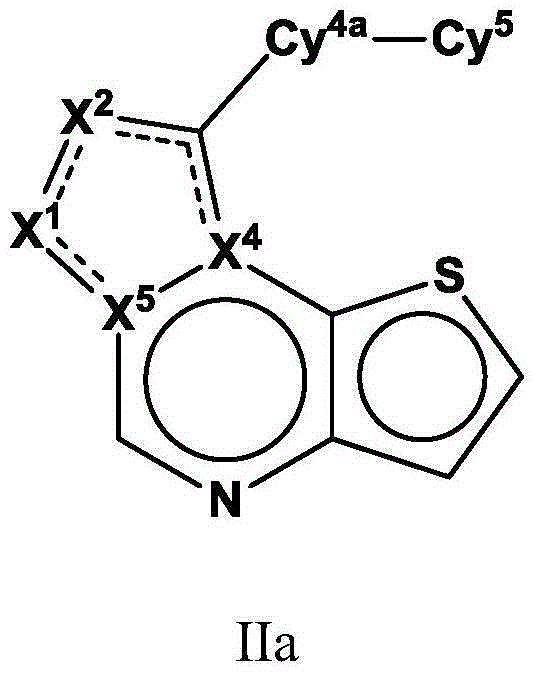
Tricyclic fused thiophene derivatives as JAK inhibitors
Technology of a compound, heterocycloalkyl, in the field of tricyclic fused thiophene derivatives as JAK inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0622] Example 1. (1R)-1-{1-[(3S)-tetrahydro-2H-pyran-3-yl]-1H-imidazo[4,5-d]thieno[3,2-b ]pyridin-2-yl}ethanol
[0623]
[0624] Step 1. 6-Nitrothieno[3,2-b]pyridin-7-ol
[0625]
[0626] N,N,N-tributylbutan-1-ammonium nitrate (from Aldrich, 9.1 g, 30 mmol) dissolved in dichloromethane (100 mL) was added dropwise to thieno[3,2- b] In a stirred solution of pyridin-7-ol (ex Aldrich, 3.0 g, 20 mmol) in dichloromethane (100 mL). Trifluoroacetic anhydride (4.5 mL, 32 mmol) was added maintaining the temperature below 0 °C. The resulting mixture was then stirred at -5°C for 30 minutes and at room temperature overnight. The reaction mixture was concentrated, diluted with ether, and filtered. The collected solids were washed successively with water and diethyl ether / methanol (MeOH) mixture (1 :1 ), and air-dried to give the desired product (3.3 g, 85%). C 7 h 5 N 2 o 3 LCMS calculated value of S: (M+H) + : m / z=197.0; found value: 196.9.
[0627] Step 2. 7-Chloro-6-nit...
Embodiment 2
[0638] Example 2. (trans-4-{2-[(1R)-1-hydroxyethyl]-1H-imidazo[4,5-d]thieno[3,2-b]pyridin-1-yl }cyclohexyl)acetonitrile
[0639]
[0640] Step 1. {trans-4-[(6-nitrothieno[3,2-b]pyridin-7-yl)amino]cyclohexyl}methanol
[0641]
[0642] 7-Chloro-6-nitrothieno[3,2-b]pyridine (0.21 g, 0.98 mmol) (Example 1, Step 2), (trans-4-aminocyclohexyl)methanol were heated at 90 °C (from J&W Pharmatech, 0.25 g, 2.0 mmol) and a mixture of N,N-diisopropylethylamine (0.51 mL, 2.9 mmol) in isopropanol (3.3 mL) for 2 hours. The resulting mixture was concentrated and purified on silica gel (eluting with 0 to 60% EtOAc in hexanes) to give the desired product (0.26 g, 86%). C 14 h 18 N 3 o 3 LCMS calculated value of S: (M+H) + : m / z=308.1; found value: 308.0.
[0643] Step 2. {trans-4-[(6-nitrothieno[3,2-b]pyridin-7-yl)amino]cyclohexyl}methyl methanesulfonate
[0644]
[0645] To {trans-4-[(6-nitrothieno[3,2-b]pyridin-7-yl)amino]cyclohexyl}methanol (0.26g, 0.84mmol) and N,N-diisopropy...
Embodiment 3
[0655] Example 3. trans-4-{2-[(1R)-1-hydroxyethyl]-1H-imidazo[4,5-d]thieno[3,2-b]pyridin-1-yl} Cyclohexanol
[0656]
[0657] Step 1. trans-4-[(6-nitrothieno[3,2-b]pyridin-7-yl)amino]cyclohexanol
[0658] 7-Chloro-6-nitrothieno[3,2-b]pyridine (0.47 g, 2.2 mmol) (Example 1, Step 2), trans-4-aminocyclohexanol (from Aldrich, 0.50 g, 4.4 mmol) and a mixture of N,N-diisopropylethylamine (1.1 mL, 6.6 mmol) in isopropanol (7.4 mL) for 2 hours. The reaction mixture was concentrated and purified on silica gel (eluting with 0 to 5% MeOH in dichloromethane) to give the desired product (0.266 g, 41%). C 13 h 16 N 3 o 3 LCMS calculated value of S: (M+H) + : m / z=294.1; found value: 294.0.
[0659] Step 2. trans-4-[(6-aminothieno[3,2-b]pyridin-7-yl)amino]cyclohexanol
[0660] at room temperature in H 2 Hydrogenation of trans-4-[(6-nitrothieno[3,2-b]pyridin-7-yl)amino]cyclohexanol (50 mg, 0.2 mmol) and 10% palladium on carbon (7 mg) under balloon pressure The mixture in methanol...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com