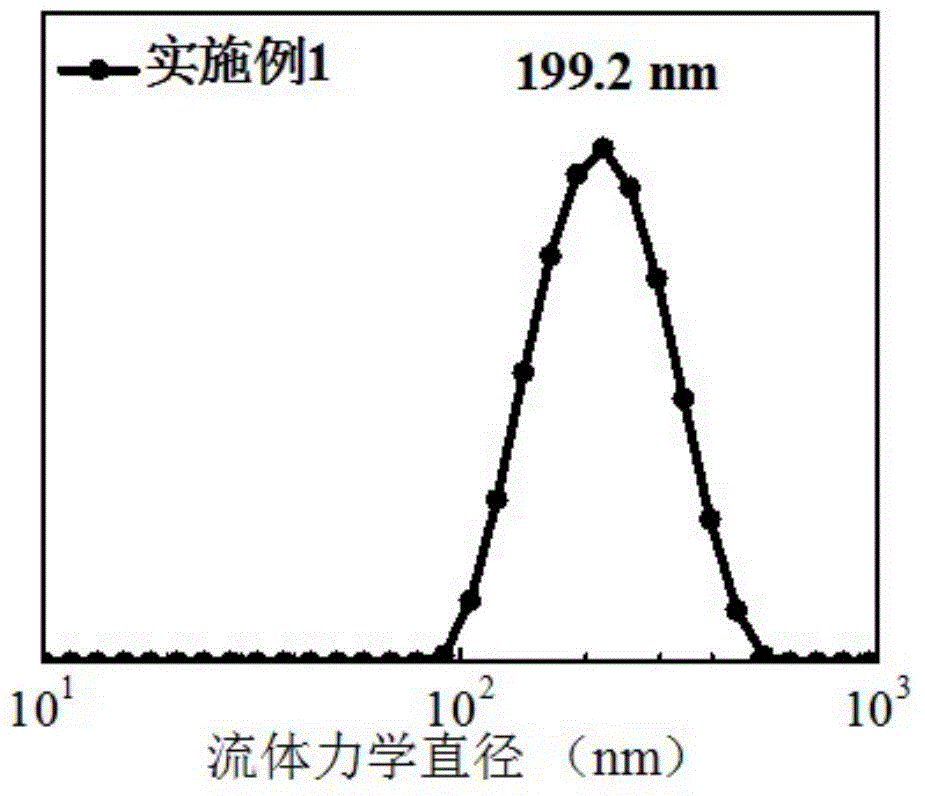
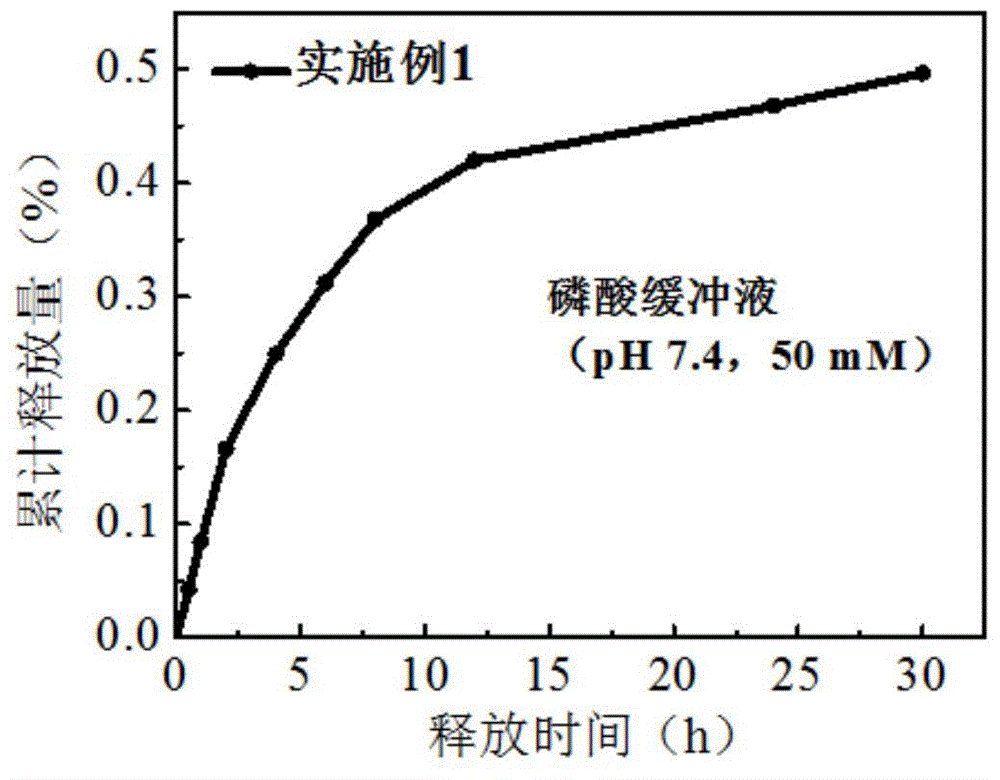
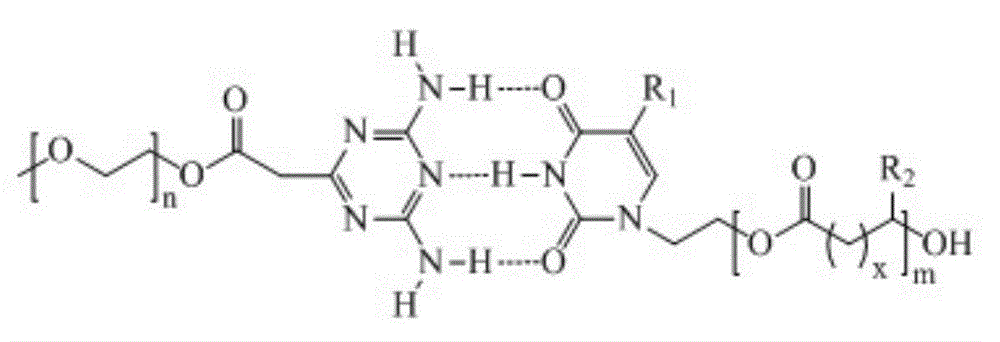
Preparation method of biodegradable supermolecule segmented copolymer and copolymer micelle
A technology of block copolymers and supramolecules, which is applied in the field of preparation of biodegradable supramolecular block copolymers and copolymer micelles, can solve the problems of weak hydrogen bonding, poor stability of block copolymers and micelles, etc. , to achieve the effects of strong triple hydrogen bond force, good versatility, and easy adjustment of structure and performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0024] Below in conjunction with accompanying drawing and specific embodiment the present invention is described in further detail:
[0025] PEG monomethyl ether and stannous octoate were purchased from Sigma-Aldrich; lactide (LA) was purchased from Praque; GA, VL, εCL, and γCL were purchased from Bailingwei; LA and GA were recrystallized and purified in ethyl acetate , VL, εCL, γCL were purified by vacuum distillation; DMF and DMSO were dried with calcium hydride for 48 hours and then used after vacuum distillation.
[0026] 1-(2-hydroxyethyl)thymine is prepared by literature (Ueda et al., Makromol.Chem.1968,120,13-20), and 1-(2-hydroxyethyl)uracil is prepared by literature (Gi etc., J .Org.Chem.1997,62,88-92) method preparation.
[0027] Step 1: Preparation of PEG-DAT
[0028] PEG-DAT was prepared according to the literature (Bayer F et al., Langmuir 2011, 27, 12851-12858). PEG (10g, 2mmol), cyanoacetic acid (0.2g, 2.4mmol), DMAP (9.8mg, 0.08mmol), DCC (0.49g, 2.4mmol) we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com