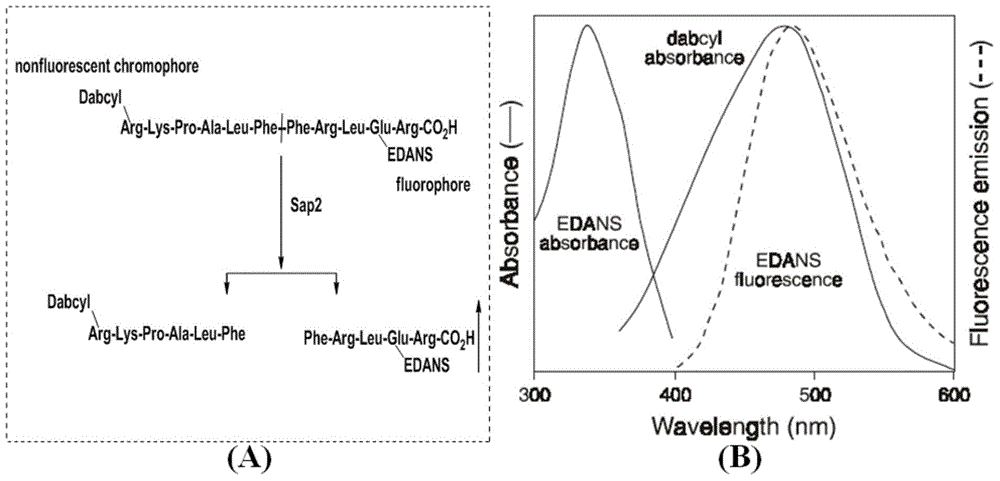
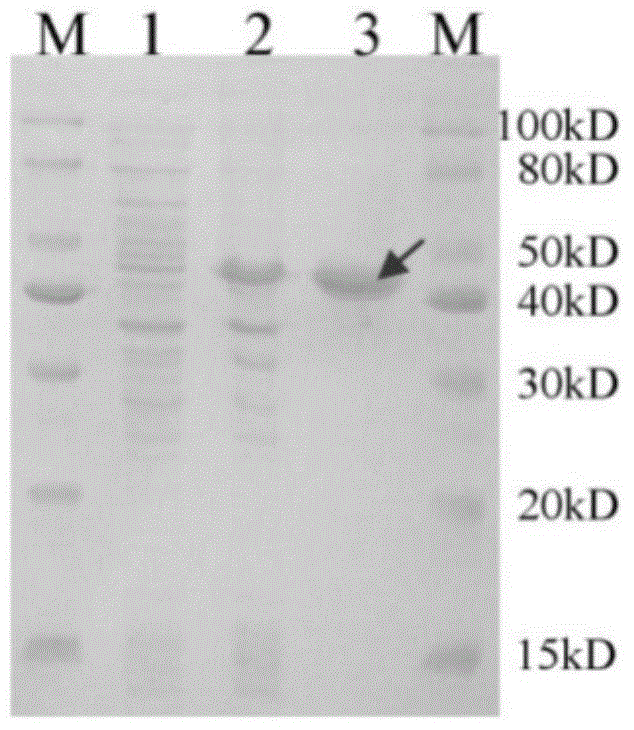
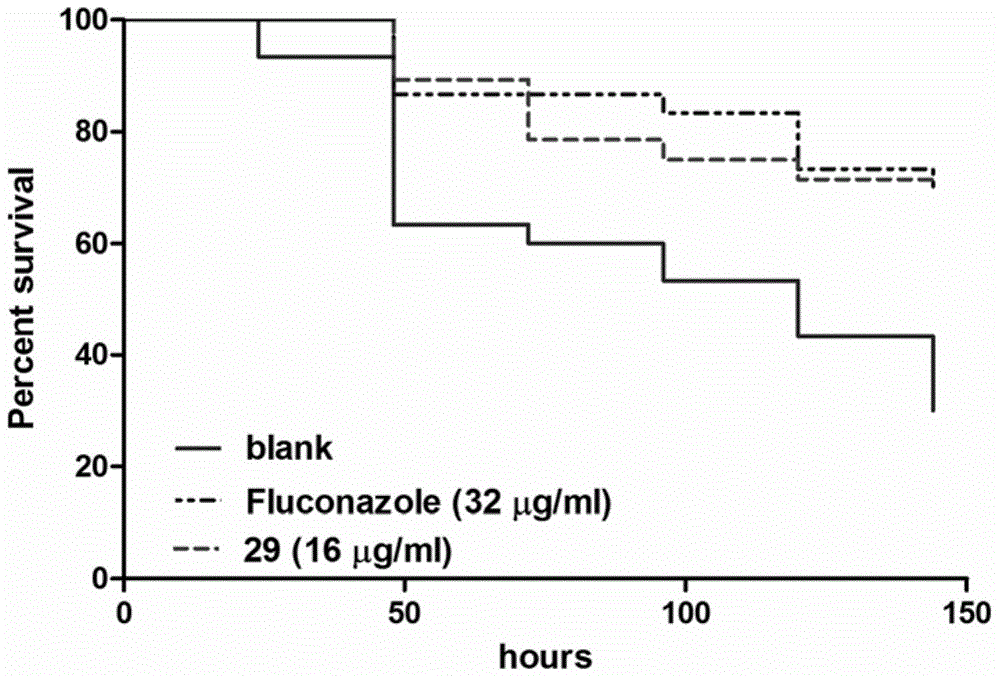
Substituted thiazole ketones secretory aspartic protease inhibitor and preparation thereof
A thiazolone, single-substitution technology, applied in the field of pharmaceutical compounds
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Example 1: Preparation of 1,3-diphenylthiourea (II, R 1 =H) Preparation
[0100] Weigh aniline (2.33g, 25mmol, 2equiv), carbon disulfide (1.24g, 16.25mmol, 2equiv), triethanolamine (2.5mL) and sulfur powder (100mg) in 50mL of water, stir and reflux for 6h. The reaction solution was cooled to room temperature, and a white precipitate precipitated out. Filtered and washed with cold water to obtain 1.46 g of white solid, yield: 51%. 1 H-NMR (300MHz, DMSO-d 6)δ:9.78(s,2H,NH),7.47(m,4H,Ar-H),7.32(m,4H,Ar-H),7.11(m,2H,Ar-H).ESI-MS(m / z):229.28[M+1].
Embodiment 2
[0101] Example 2: 3-phenyl-2-(phenylimino)thiazolidin-4-one (Ⅲ, R 1 =H) Preparation
[0102] Weigh 1,3-diphenylthiourea (1.14g, 4.0mmol, 1equiv), anhydrous sodium acetate (1.64g, 20.0mmol, 5equiv), 0.85mL ethyl chloroacetate (0.98g, 8.0mmol, 2equiv) In 20 mL of absolute ethanol, the mixture was stirred at 60° C. for 6 h. The reaction solution was cooled to room temperature, and a precipitate appeared. The precipitate was filtered, washed with ethanol, and recrystallized with ethyl acetate to obtain 0.68 g of an orange solid, yield: 64%, which was directly used in the next reaction without further purification. 1 H-NMR (300MHz, DMSO-d 6 )δ:7.47-7.59(m,2H),7.37-7.46(m,3H),7.25-7.36(m,2H),7.08(t,1H,J=7.8Hz),6.81-6.92(m,2H) ,4.15(s,2H).ESI-MS(m / z):269.35[M+1].
Embodiment 3
[0103] Example 3: Ethyl 2-(2-{[4-oxo-3-phenyl-2-(phenylimino)thiazolidin-5-enylidene]methyl}phenoxy)acetate (IV, R 1 =H) Preparation
[0104] Weigh 3-phenyl-2-(phenylimino)thiazolidin-4-one (0.3g, 1.12mmol, 1equiv), ethyl 2-formylphenoxy acetate (0.28g, 1.35mmol, 1.2equiv) , piperidine (0.095g, 1.12mmol, 1equiv) in 5mL of absolute ethanol, the mixture was stirred at 60°C for 8h. Purification by flash preparative chromatography (PE-EtOAc, 5:1) gave 0.48 g of light yellow solid, yield: 95%. 1 H-NMR (300MHz, DMSO-d 6 )δ:8.07(s,1H),7.44-7.62(m,5H),7.30-7.43(m,4H),7.02-7.22(m,3H),6.90-7.01(m,2H),4.94(s, 2H), 4.16(dd, 2H, J=7.06, 14.02Hz), 1.20(t, 3H, J=7.31Hz). ESI-MS (m / z): 459.40[M+1].
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com