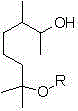
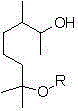
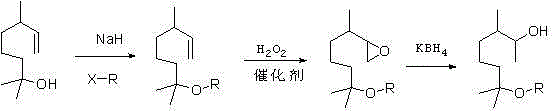Methoxy elgenol derivative and preparation method thereof
The technology of a derivative and sandalwood ether, applied in the field of sandalwood ether derivatives and their preparation, can solve the problems of high use cost of natural sandalwood oil, scarcity of natural sandalwood resources, etc., and achieves high yield, environmental friendliness, and applicability. wide range of effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] (1) Synthesis of 3,7-dimethyl-7-butoxyoctene
[0026] At room temperature, add 400ml of toluene into a 1000ml three-necked flask, add myrcenol (156.1g, 1mol) in turn under stirring, add 60% sodium hydride (48g, 1.2mol) in batches, increase the temperature to 110 degrees, and keep it warm for 2 hours. 1-Chlorobutane (92.6g, 1mol) was added dropwise and the reaction was continued for 6 hours, cooled to room temperature, quenched with ice water, the organic layer was separated, and the organic layer was rectified under reduced pressure to recover 41.2g of raw material myrcenol. The collected product fraction 142.4g (gas content 95.8) yield 67.2%.
[0027] (2) Synthesis of 3,7-dimethyl-7-butoxy-1,2-epoxyoctane
[0028] The catalyst for the epoxidation reaction has been disclosed in CN00123339. 4, and its preparation process is shown in CN00123339. 4.
[0029] Add 80 g (2.32mol) 30% hydrogen peroxide and 98.4 g (0.464 mol) 3,7-dimethyl-7-butoxy-1,2-epoxyoctane into a 500 mL three-n...
Embodiment 2
[0033] (1) Synthesis of 3,7-dimethyl-7-pentoxyoctene
[0034] At room temperature, add 400ml of toluene into a 1000ml three-necked flask, add myrcenol (156.1g, 1mol) in turn while stirring, add 60% sodium hydride (60g, 1.5mol) in batches, increase the temperature to 110 degrees, and keep it warm for 2 hours. Add 1-chloropentane (106.6g, 1mol) dropwise and continue to stir and react for 6 hours, cool to room temperature, add ice water to quench the reaction, separate the organic layer, and rectify the organic layer under reduced pressure to recover 36g of raw material myrcenol and collect The product fraction is 239.2g (gas content 96.3), the yield is 75.1%.
[0035] (2) Synthesis of 3,7-dimethyl-7-butoxy-1,2-epoxyoctane
[0036] The catalyst for the epoxidation reaction has been disclosed in CN00123339. 4, and its preparation process is shown in CN00123339. 4.
[0037] Add 80 g (2.32mol) 30% hydrogen peroxide and 147.6g (0.464 mol) 3,7-dimethyl-7-pentyloxy-1,2-epoxyoctane into a 500 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com