S-triazolo-thiadiazole and thiadiazine derivatives, preparation method and application thereof
A technology of thiadiazines and derivatives, applied in the field of medicine, can solve the problems that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
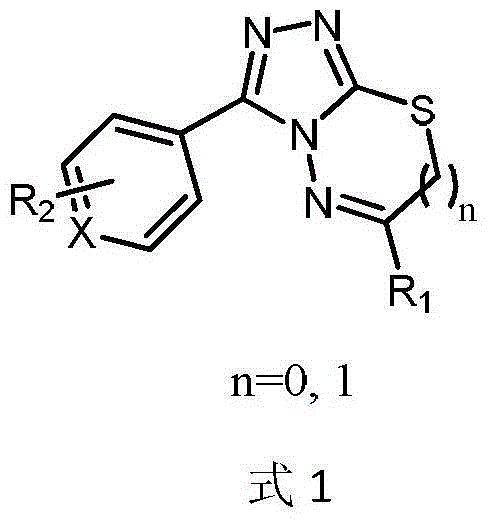
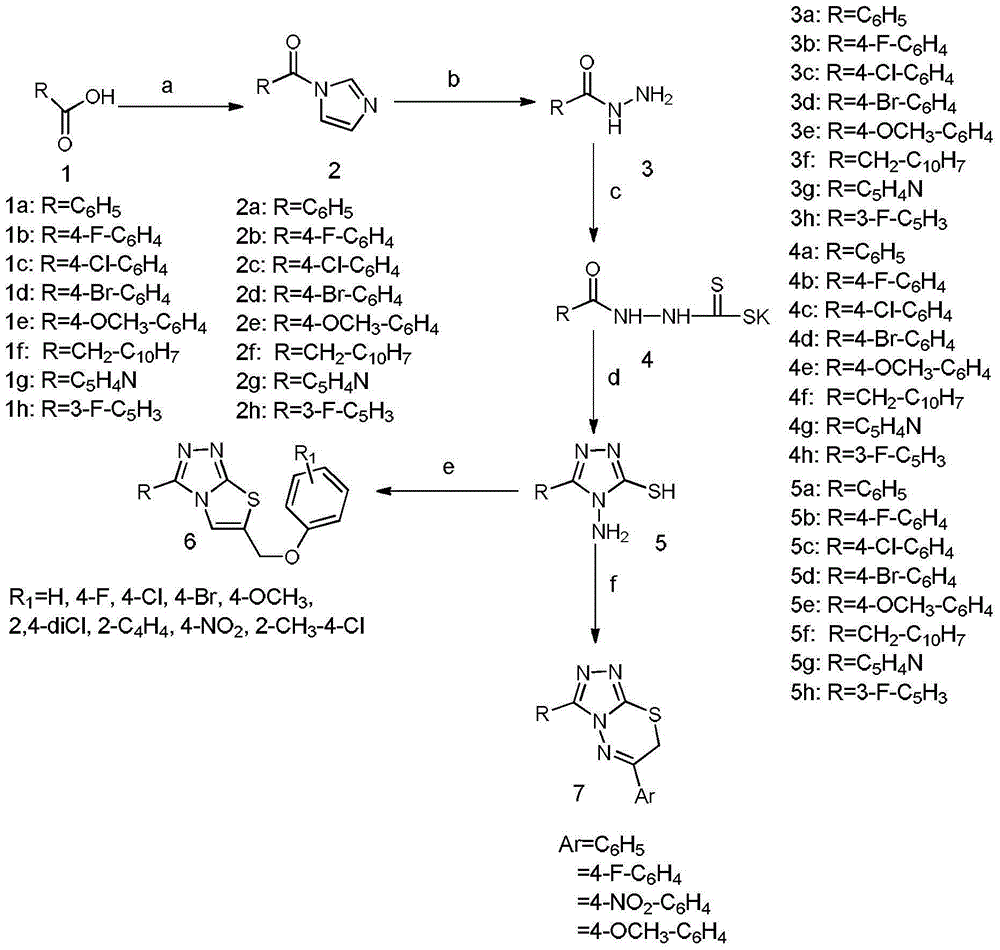
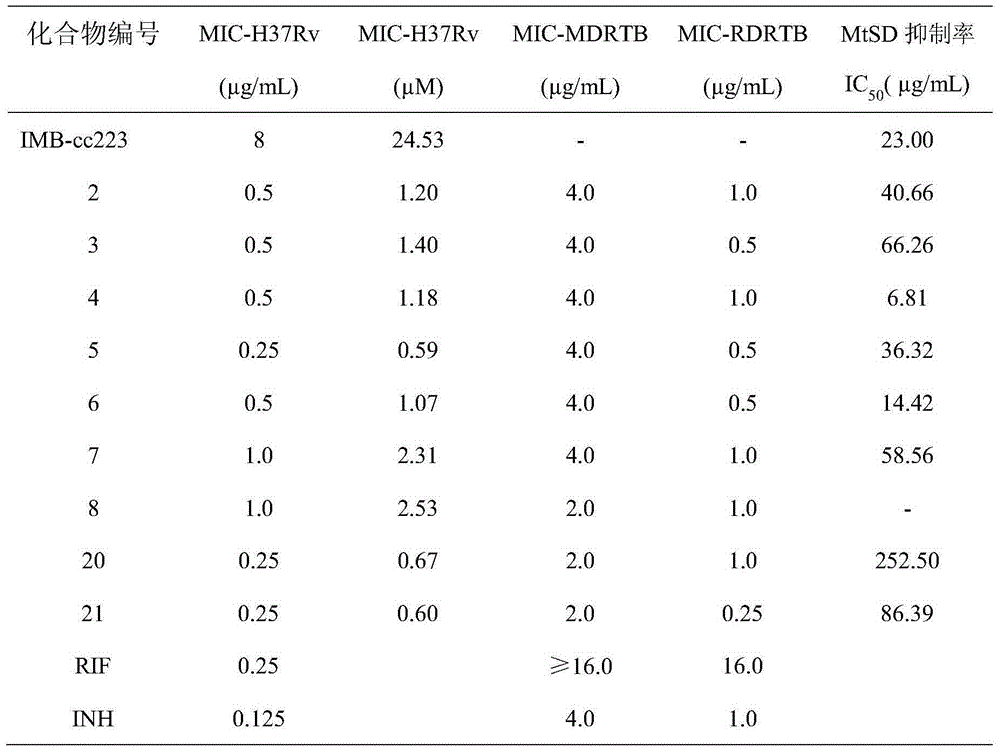
[0069] "Example 1" 3-(4-fluorophenyl)-6-(phenoxymethyl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadi The synthesis of azole (compound 1 is: IMB-CC223) is divided into the following steps:
[0070] 1) Synthesis of p-fluorobenzoic hydrazide (intermediate 3)
[0071] Add p-fluorobenzoic acid (10.0g, 60mmol) and 100mL tetrahydrofuran in a 250mL three-necked flask, then add N,N'-carbonyldiimidazole (12.6g, 78mmol), TLC monitors whether the reaction of the raw materials is complete, after the completion of the reaction, slowly 80% hydrazine hydrate (5.94 g, 95 mmol) was added dropwise, and the reaction was stirred for about 6 hours after the drop was completed. TLC monitors whether the reaction of raw materials is complete (developing solvent: petroleum ether / ethyl acetate 1:1). After completion of the reaction, cool to room temperature, concentrate under reduced pressure to remove most of the organic solvent, add about 30 mL of water, beat at room temperature for a while, filter with suct...
Embodiment 2
[0078] "Example 2" 3-(4-chlorophenyl)-6-(4-bromophenoxymethyl)-[1,2,4]triazolo[3,4-b][1,3,4 Synthesis of ]thiadiazole (compound 2)
[0079] Change p-fluorobenzoic acid into 2-naphthylacetic acid, and phenoxyacetic acid into p-nitrophenoxyacetic acid, and use a method similar to "Example 1" to obtain 3-(2-naphthylmethyl)-6-(4- Nitrophenoxymethyl)-[1,2,4]triazolo[3,4-b][1,3,4]thiadiazole (Compound 2). 1 H NMR (500MHz, DMSO-d6) δ8.27–8.21(m,3H),7.97–7.93(m,1H),7.86(d,J=7.7Hz,1H),7.55(m,2H),7.46– 7.40 (m, 2H), 7.31–7.27 (m, 2H), 5.70 (s, 2H), 4.87 (s, 2H). HRMS-APCI: Calculated C 21 h 15 N 5 o 3 S[M+H] + : 418.0968; actual value: 418.0953.
Embodiment 3
[0080]"Example 3" 3-(4-fluorophenyl)-6-(4-methoxyphenoxymethyl)-[1,2,4]triazolo[3,4-b][1,3 ,4] Synthesis of Thiadiazole (Compound 3)
[0081] Phenoxyacetic acid is replaced with p-methoxyphenoxyacetic acid, and a method similar to "Example 1" is used to obtain 3-(4-fluorophenyl)-6-(4-methoxyphenoxymethyl)-[ 1,2,4]triazolo[3,4-b][1,3,4]thiadiazole (Compound 3). 1 H NMR (400MHz, DMSO-d6) δ8.26(d, J=8.8Hz, 2H), 7.48(d, J=8.8Hz, 2H), 7.08(d, J=9.0Hz, 2H), 6.91(d , J=9.0Hz, 2H), 5.56(s, 2H), 3.71(s, 3H). HRMS-APCI: Calculated C 17 h 13 FN 4 o 2 S[M+H] + : 357.0816; actual value: 357.0823.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com