Preparation method of quinoxaline compounds
A compound, the technology of quinoxaline, which is applied in the field of preparation of quinoxaline compounds, can solve the problems such as the limitation of synthetic molecular structure diversity, and achieve the effect of diverse structure of target products, simple and easy synthesis process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] Dissolve 1.0 mmol of 1,3-dicarbonyl compound and 1.0 mmol of 2,2,6,6-tetramethylpiperidine oxide in 20 mL of ethyl acetate, add 0.2 mmol of cerium ammonium nitrate and heat to 60 °C and stir for 30 Minutes, after the reaction is complete, filter to remove insoluble inorganic salts, remove ethyl acetate by rotary evaporation, and separate by column chromatography (eluent: petroleum ether: ethyl acetate = 20:1) to obtain α-position 2,2,6,6-tetramethyl 1,3-dicarbonyl compounds substituted by base piperidine oxides, the 1,3-dicarbonyl compounds substituted by α-position 2,2,6,6-tetramethylpiperidinium oxides are used for quinoxaline compounds synthesis.
Embodiment 2
[0020]
[0021] Dissolve 108 mg (1.0 mmol) of the raw material compound o-phenylenediamine and 314 mg (1.1 mmol) of ethyl acetoacetate substituted by α-position TEMPO in 5.0 mL of glacial acetic acid, stir the reaction system at room temperature for 10 minutes, evaporate the acetic acid, add The residue was dissolved in ethyl acetate, washed twice with saturated sodium bicarbonate solution (30mL×2), the organic layer was separated, dried over anhydrous sodium sulfate, filtered, and the solvent was removed by rotary evaporation, column chromatography (eluent: petroleum ether: acetic acid Ethyl ester=5:1) isolated compound 3-1 Pure product 212mg (98%).
[0022] 1 H NMR (400 MHz, CDCl 3 ) δ 8.19 (dd, J = 8.3, 1.2 Hz, 1H), 8.05 (d, J = 8.3 Hz, 1H), 7.83 (ddt, J = 8.7, 7.0, 1.7 Hz, 1H), 7.77 (ddd, J = 8.7, 3.3, 1.7 Hz, 1H), 4.57 (qd, J = 7.1, 1.4 Hz, 2H), 2.96 (d, J = 1.5 Hz, 3H), 1.50 (td, J = 7.1, 1.5 Hz, 3H).
Embodiment 3
[0024]
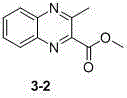
[0025] In addition to using α-position TEMPO-substituted methyl acetoacetate instead of α-position TEMPO-substituted ethyl acetoacetate, in order to prepare the compound 3-1 Compounds are prepared in the same way 3-2 .
[0026] 1 H NMR (400 MHz, CDCl 3 ) δ 8.19 (dd, J = 8.4, 1.1 Hz, 1H), 8.05 (dd, J = 8.4, 1.0 Hz, 1H), 7.87-7.81 (m, 1H), 7.80-7.73 (m, 1H), 4.09 (s, 3H), 2.98 (s, 3H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com