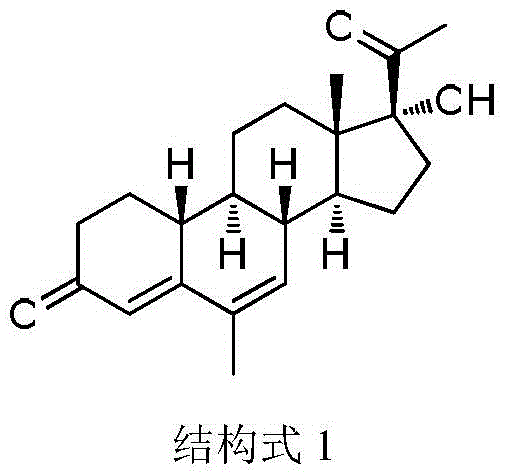
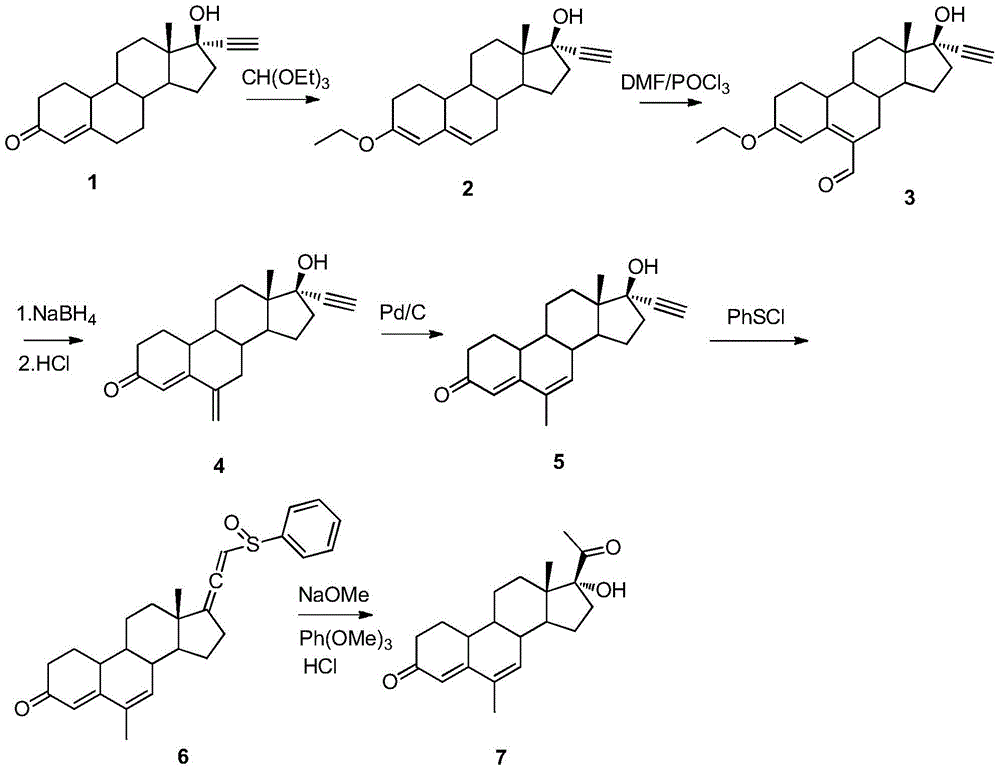
A method for synthesizing 6-methyl-17α-hydroxyl-19-norpregna-4,6-diene-3,20-dione
A technology of methyl-pregnant and norpregnant, which is applied in the direction of steroidal compounds and organic chemistry, can solve problems such as difficulty in obtaining, achieve high yield, simple reaction operation, and reduce the possibility of metal residues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] 1.1 3-Ethoxy-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (2)
[0033] Compound 1 (15g, 0.05mol) was dissolved in THF (150ml) and added to the reaction flask, then triethyl orthoformate (14.8g, 0.1mol) and p-toluenesulfonic acid (0.15g, 0.8mmol) were added. The reaction solution was stirred at 25°C for 12 hours, pyridine (3ml) was added, most of the solvent was evaporated under reduced pressure, cooled and filtered, and dried to obtain 15g of the product with a yield of 92%. HNMR (ppm, CDCl 3 ):5.65(s,1H),4.73(s,1H),3.72-3.82(q,2H),3.35(s,1H),3.14(b,1H),2.20-2.60(m,5H),1.80- 2.10(m,6H),1.20-1.60(m,8H),0.95-1.05(m,2H),0.72(s,3H).
[0034] 1.2 3-Ethoxy-6-formyl-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (3)
[0035] Add compound 2 (14g, 0.043mol), DMF (100ml) into the reaction flask, stir to dissolve, cool to -20°C, add Vilsmeier reagent dropwise, react for 15 minutes, pour the reaction solution into saturated sodium bicarbonate solution (400ml), suction filtratio...
Embodiment 2
[0045] 2.1 3-Ethoxy-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (2)
[0046] Compound 1 (15g, 0.05mol) was dissolved in THF (150ml) and added to the reaction flask, then triethyl orthoformate (14.8g, 0.1mol) and p-toluenesulfonic acid (0.15g, 0.8mmol) were added. The reaction solution was stirred at 80° C. for 3 hours, pyridine (3 ml) was added, the solvent was evaporated under reduced pressure, filtered with suction, and dried to obtain 13.5 g of the product with a yield of 83%. The product analysis result is consistent with the product obtained in Example 1.1.
[0047] 2.2 3-Ethoxy-6-formyl-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (3)
[0048] Compound 2 (13g, 0.040mol), DMF (100ml) was added to the reaction flask, stirred and dissolved, cooled to 0°C, Vilsmeier reagent was added dropwise, reacted for 15 minutes, and the reaction solution was poured into saturated sodium bicarbonate solution ( 400ml), 12.8g of the product was obtained after recrystallization, and ...
Embodiment 3
[0058] 3.1 3-Ethoxy-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (2)
[0059] Compound 1 (15g, 0.05mol) was dissolved in THF (150ml) and added to the reaction flask, then triethyl orthoformate (14.8g, 0.1mol) and p-toluenesulfonic acid (0.15g, 0.8mmol) were added. The reaction solution was stirred at 60° C. for 4 hours, pyridine (3 ml) was added, the solvent was removed under reduced pressure, and the product was dried to obtain 14.3 g with a yield of 87%. The product analysis result is consistent with the product obtained in Example 1.1.
[0060] 3.2 3-Ethoxy-6-formyl-17α-ethynyl-19-desmethylpregna-3,5-dien-17β-ol (3)
[0061] Add compound 2 (14g, 0.043mol), DMF (100ml) into the reaction flask, stir to dissolve, cool to 0-5°C, add Vilsmeier reagent dropwise, react at 5-10°C for 10 minutes, pour the reaction solution into In saturated sodium bicarbonate solution (300ml), 12.2g of the product was obtained after recrystallization, with a yield of 80%. The product analysis r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com