Potassium magnesium aspartate injection and preparation method thereof
A technology of potassium magnesium aspartate and potassium aspartate, applied in the field of medicine, can solve problems such as precipitation of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-5
[0032] Add water for injection of 2 / 3 of the total volume of the prepared medicinal solution, add 400 g of magnesium aspartate, and stir until dissolved. Add 450 g of potassium aspartate and stir until dissolved. Weigh 5 g of activated carbon, add it to the above solution, and stir for 15 min. Add water for injection to 10000ml, stir well. Store the filtered medicinal solution at 25-40°C. Fill and seal the filtered medicinal solution in glass ampoules. The intermediate product after potting is sterilized (121°C for 15min) and leak tested.
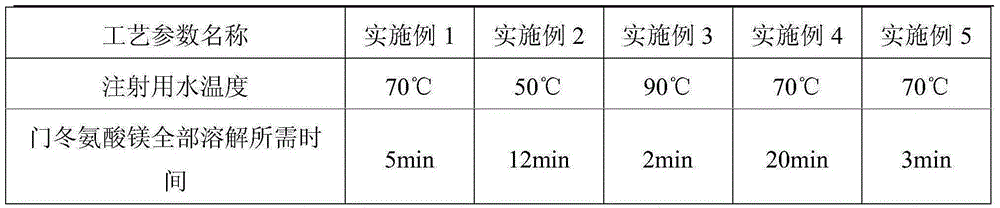
[0033] See Table 1 for the stirring speeds of adding magnesium aspartate and potassium aspartate and the time required for the complete dissolution of magnesium aspartate in Examples 1-5.
[0034] Table 1 embodiment 1-5 stirring speed and magnesium aspartate all dissolve required time
[0035]
[0036]
[0037] It can be seen from the data in Table 1 that the preferred process parameters are: stirring speed 100 rpm, temperature o...
Embodiment 6-10
[0039] Add 70°C water for injection of 2 / 3 of the total volume of the prepared drug solution, add 400 g of magnesium aspartate, and stir at 100 rpm for 15 min. Add 450 g of potassium aspartate and stir until dissolved. Take the gac of embodiment amount, add in the above-mentioned solution, stir. Add water for injection to 10000ml, stir well. Store the filtered medicinal solution at 25-40°C. Fill and seal the filtered medicinal solution in glass ampoules. The intermediate product after potting is sterilized (121°C for 15min) and leak tested.
[0040] See Table 2 for activated carbon consumption and time in Examples 6-10.
[0041] Active carbon consumption and time in table 2 embodiment 6-10
[0042] project
[0043] The purpose of adding activated carbon in the production process of injections is to adsorb pyrogens and decolorize. If the amount is too small, it may not be able to remove pyrogens and decolorize. If the amount is too large, it may cause drug adsor...
Embodiment 11-13
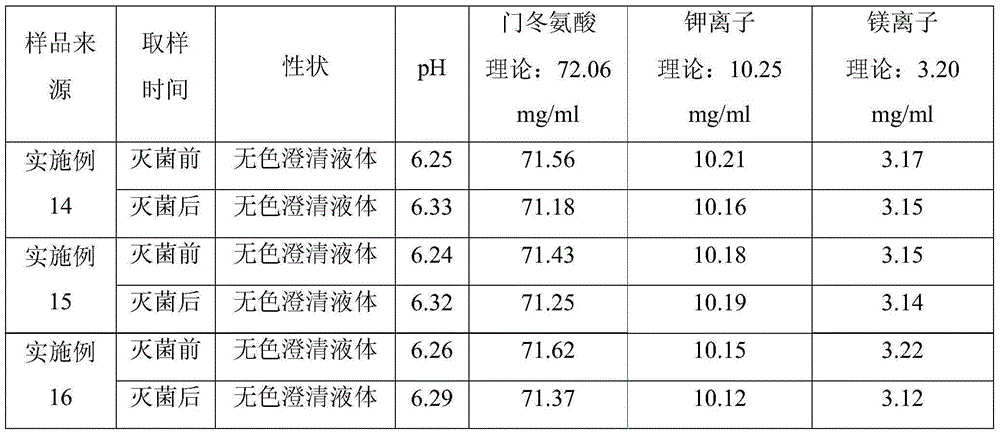
[0045] Add 2 / 3 of the total volume of the prepared drug solution with 70°C water for injection, add 400 g of magnesium aspartate, and stir at 100 rpm for 10 min. Add 450 g of potassium aspartate and stir until dissolved. Weigh 5 g of activated carbon, and add it into the above solution, and stir. Add water for injection to 10000ml, stir well. In order to facilitate the storage of the medicinal solution at different temperatures, the medicinal solution was filled in 10ml glass ampoules, and 200 ampoules were stored at each temperature.
[0046] See Table 3 for the solution storage temperature and time during the filling process of Examples 11-13.
[0047] As can be seen from the data in Table 3, the storage temperature of the potassium magnesium aspartate solution during the filling process is 25-40°C, and the preferred storage temperature is 30°C.
[0048] Solution storage temperature and time in the filling process of table 3 embodiment 11-13
[0049] project
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com