Bortezomib-containing medicinal composition and preparation method thereof
The technology of bortezomib and composition is applied in the field of bortezomib-containing composition and preparation thereof, which can solve the problems of easy-to-oxidize freeze-dried powder injection, difficulty in dissolving bortezomib and high water content, and achieve low cost and stable quality , The effect of simple operation of the production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
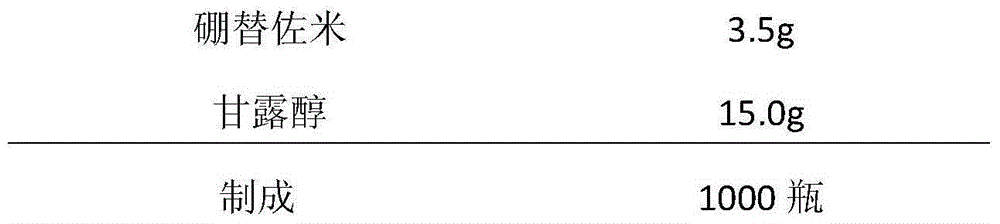
[0037] prescription:
[0038]
[0039]
[0040] Process:
[0041] (1) Add mannitol to 700ml water for injection, stir until completely dissolved;
[0042] (2) Bortezomib is added to 160ml of tert-butanol solution and stirred until completely dissolved;
[0043] (3) Mix the above two solutions evenly, filter and fill the medicinal solution with a 0.22 μm filter;
[0044] (4) Pre-freezing: Pre-freezing at -45°C for 4 hours;
[0045] (5) Sublimation drying 1: heat up to -35°C in 1h, keep for 10h, vacuum degree 0.06mbar;
[0046] (6) Sublimation drying 2: heat up to 0°C in 20 hours, vacuum degree 0.12mbar;
[0047] (7) Analytical drying: heat up to 40°C in 2 hours, keep for 4 hours, and vacuum degree 0.01mbar;
[0048] (8) Full pressure plug, rolled aluminum plastic cover.
Embodiment 2
[0050] prescription:
[0051]
[0052] Process:
[0053] (1) Add mannitol to 300ml water for injection, stir until completely dissolved;
[0054] (2) Add bortezomib to 87.5ml tert-butanol solution, stir until completely dissolved;
[0055] (3) Mix the above two solutions evenly, filter and fill the medicinal solution with a 0.22 μm filter;
[0056] (4) Pre-freezing: Pre-freezing at -50°C for 3 hours;
[0057] (5) Sublimation drying 1: heat up to -30°C in 1h, keep for 5h, vacuum degree 0.12mbar;
[0058] (6) Sublimation drying 2: heat up to 0°C in 4 hours, vacuum 0.06mbar;
[0059] (7) Analytical drying: heat up to 20°C in 1h, keep for 8h, vacuum degree 0.001mbar;
[0060] (8) Full pressure plug, rolled aluminum plastic cover.
Embodiment 3
[0062] prescription:
[0063]
[0064] Process:
[0065] (1) Add mannitol to 420ml water for injection, stir until completely dissolved;
[0066] (2) Bortezomib is added to 280ml of tert-butanol solution and stirred until completely dissolved;
[0067] (3) Mix the above two solutions evenly, filter and fill the medicinal solution with a 0.22 μm filter;
[0068] (4) Pre-freezing: Pre-freezing at -50°C for 5 hours;
[0069] (5) Sublimation drying 1: raise the temperature to -33°C in 2 hours, keep it for 8 hours, and vacuum degree 0.10mbar;
[0070] (6) Sublimation drying 2: heat up to 0°C for 8 hours, vacuum 0.10mbar;
[0071] (7) Analytical drying: heat up to 45°C for 6 hours, keep for 6 hours, and vacuum degree 0.004mbar;
[0072] (8) Full pressure plug, rolled aluminum plastic cover.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com