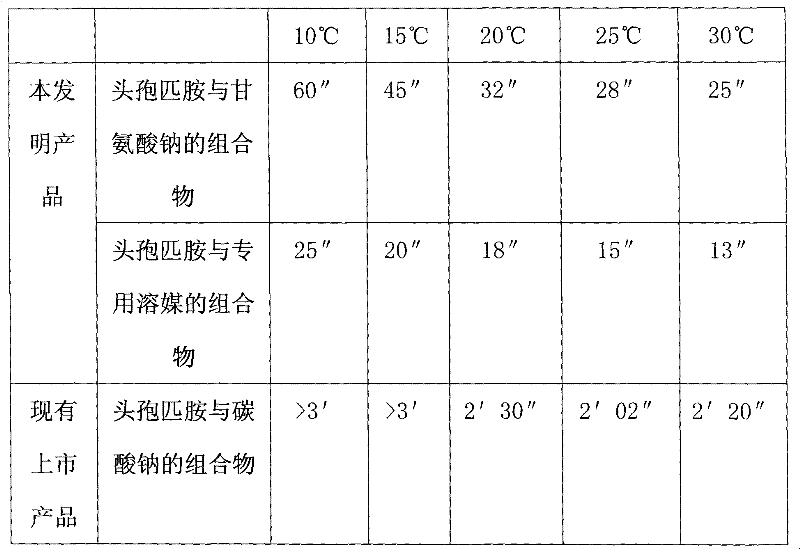
Composition of cefpiramide and sodium glycinate or special solvent composition containing sodium glycinate
A technology of sodium glycinate and cefpiramide, applied in the field of medicine, can solve the problems of slow dissolution, caking, difficult operation, etc., and achieve the effect of improving resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The composition of embodiment 1 cefpiramide and sodium glycinate
[0026] Weigh 150.0 g of cefpiramide, mix it with 22.8 g of sodium glycinate, and after mixing for 1 hour, pack it according to the specification of 0.5 g / bottle, and squeeze the cap to get it.
Embodiment 2
[0027] The composition of embodiment 2 cefpiramide and sodium glycinate
[0028] Weigh 150.0 g of cefpiramide, mix it with 24.9 g of sodium glycinate, and after mixing for 1 hour, pack it according to the specification of 0.5 g / bottle, and squeeze the cap to get it.
Embodiment 3
[0029] The composition of embodiment 3 cefpiramide and sodium glycinate
[0030] Weigh 100.0 g of cefpiramide, mix it with 18.1 g of sodium glycinate, and after mixing for 1 hour, pack it according to the specification of 0.5 g / bottle, and squeeze the cap to get it.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com