Preparation method and application of high selectivity ultrasensitive inorganic mercury / organic mercury ion fluorescent probe
A preparation, hydrogen atom technology, applied in organic chemistry, fluorescence/phosphorescence, chemical instruments and methods, etc., can solve the problems of poor water solubility, complex synthesis, poor selectivity, etc., and achieve the effect of good stability and simple synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039]
[0040] (Scheme 1) 534 mg (2.0 mmol) of N-butyl-4-hydroxy-1,8-naphthalimide, 247 mg (2.0 mmol) of dimethylaminothioformyl chloride and (250 μL) of N,N-diiso Propylethylamine (DIPEA) was dissolved in 15 mL of dichloromethane, stirred and reacted at 25°C for 12 h, then rotary evaporated to obtain a crude product, and finally recrystallized using dichloromethane and petroleum ether to obtain 326 mg of a pure product with a yield of 46 ﹪.
[0041] (Scheme 2) 534 mg (2.0 mmol) of N-butyl-4-hydroxy-1,8-naphthalimide, 494 mg (4.0 mmol) of dimethylaminothioformyl chloride and (350 μL) of N,N-diiso Propylethylamine (DIPEA) was dissolved in 15 mL of dichloromethane, stirred and reacted at 25°C for 12 h, then rotary evaporated to obtain a crude product, and finally recrystallized using dichloromethane and petroleum ether to obtain 411 mg of a pure product with a yield of 58 ﹪.
[0042](Scheme 3) 534 mg (2.0 mmol) of N-butyl-4-hydroxy-1,8-naphthalimide, 618 mg (6.0 mmol) of d...
Embodiment 2
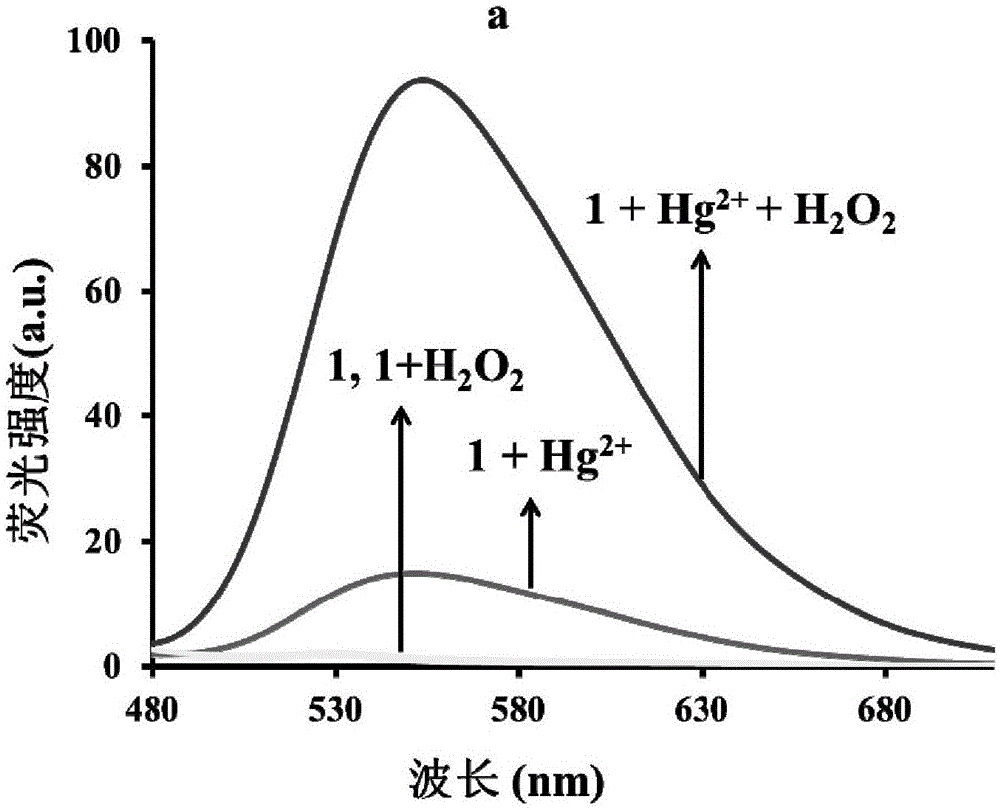
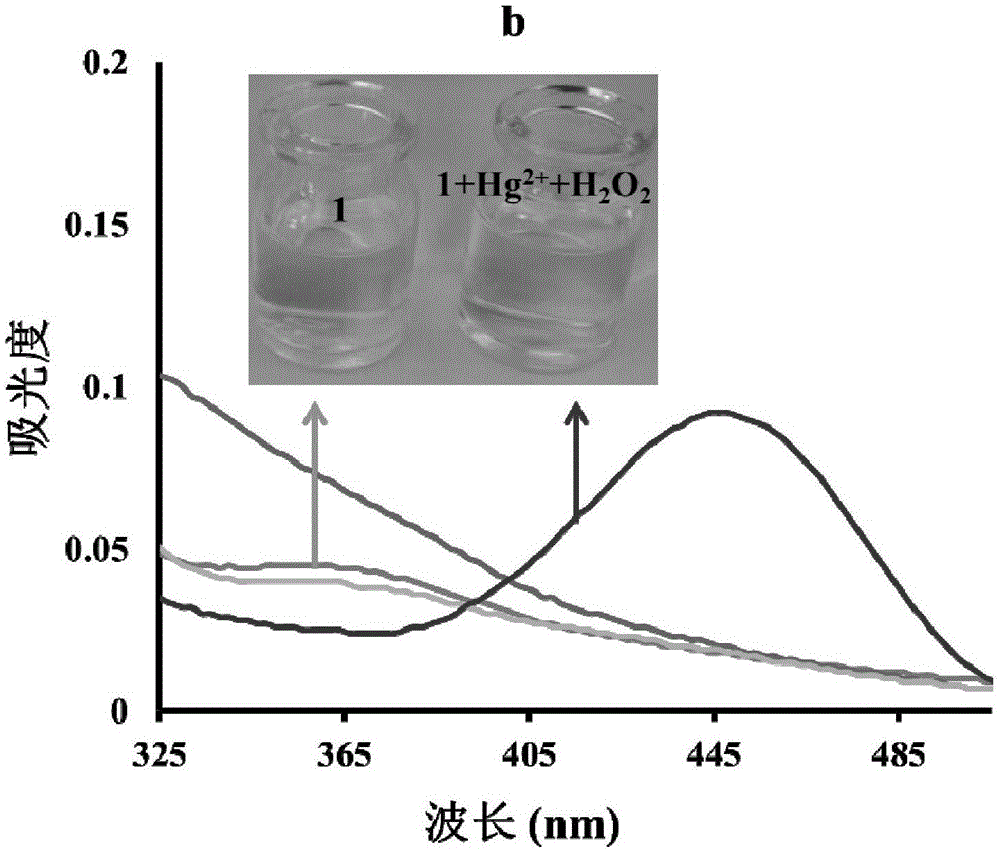
[0047] The inventors of the present invention have carried out following test: (a) Hg 2+ (10μM) in H 2 o 2 (100mM) in the presence of probe (5μM) fluorescence spectrum; (b) Hg 2+ (30μM) in H 2 o 2 Effect of the presence of (100 mM) on the UV spectrum of the probe (5 μM). The above determination is carried out in 5mM HEPES, pH7.4 aqueous solution, the probe used is the probe prepared in Example 1, and all spectral tests are Hg at 25°C 2+ Measured after 30 minutes of adding action. See Figure 1 for the results.
[0048] From Figure 1(a), it can be seen that in H 2 o 2 In the presence of Hg 2+ The fluorescence intensity is greatly enhanced; as can be seen from Figure 1(b), at H 2 o 2 In the presence of , the absorption peak was red-shifted obviously, and the color of the solution changed from colorless to yellow-green.
Embodiment 3
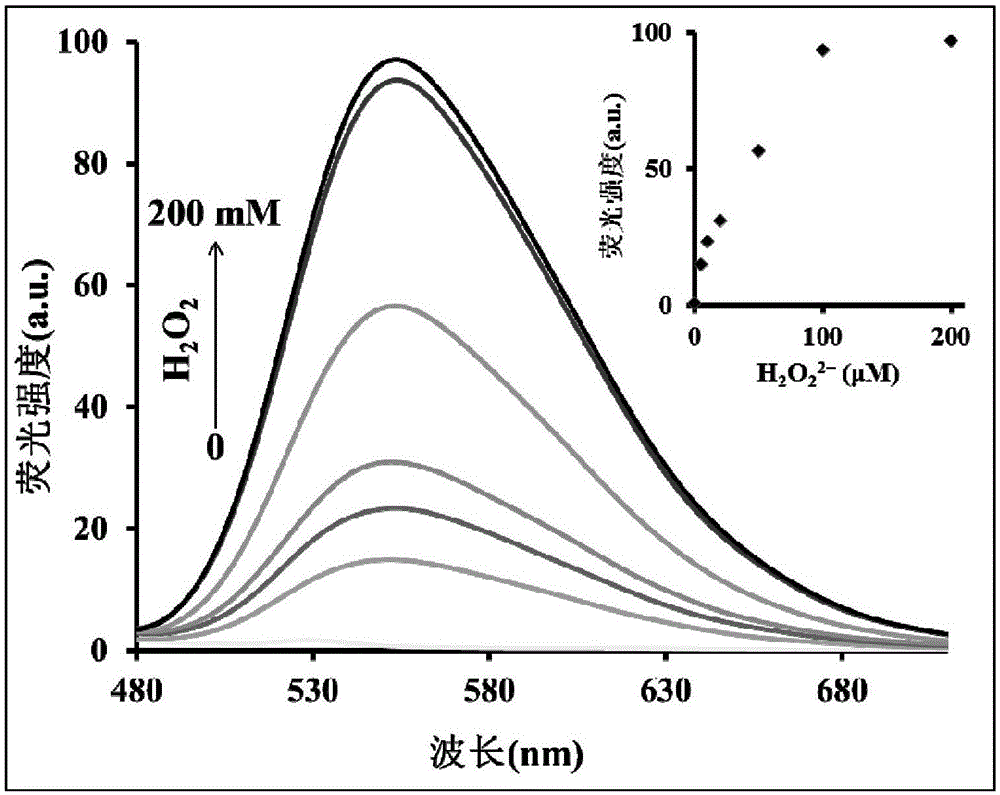
[0050] Hg 2+ (10μM) at different concentrations of H 2 o 2 Effect of presence on the fluorescence spectrum of the probe (5 μM). The above determination is carried out in 5mM HEPES, pH7.4 aqueous solution, the probe used is the probe prepared in Example 1, and all spectral tests are Hg at 25°C 2+ Measured after 30 minutes of adding action. See results figure 2 .
[0051] from figure 2 It can be seen that with the H in the probe solution 2 o 2 As the concentration increases, the fluorescence spectrum increases gradually. From this it can be concluded that H 2 o 2 The optimal concentration taken should be 100mM.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com