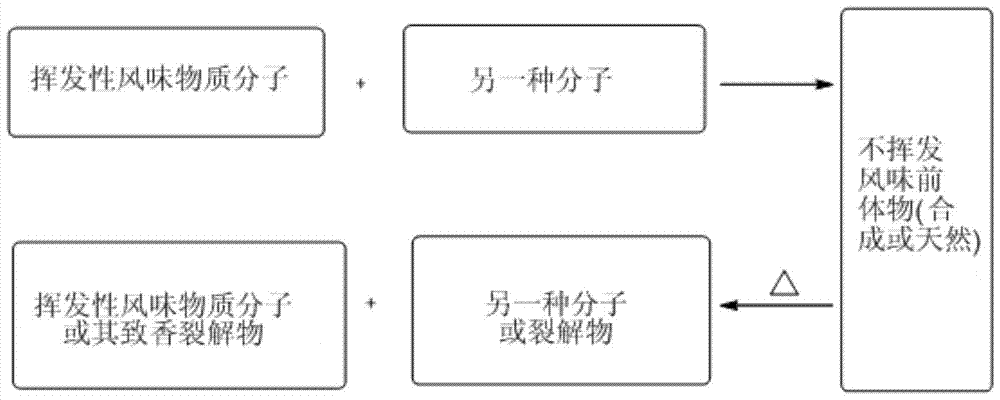
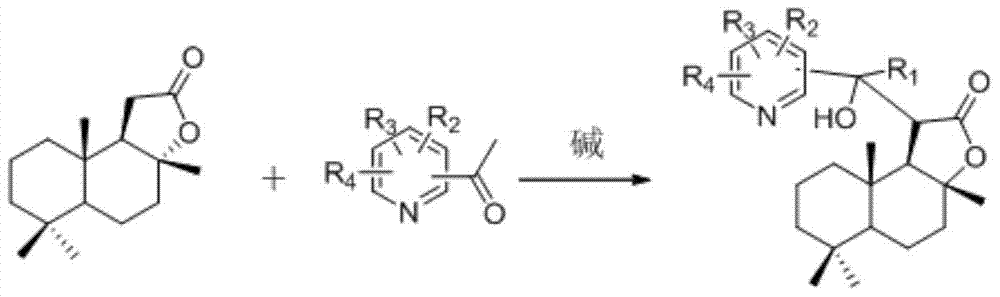
Latent fragrance compound based on ambroxolactone and its preparation method and application
A technology for ambroxolide and compound, which is applied in the field of tobacco flavor, can solve the problems of inability to use tobacco formulations well, unstable cigarette quality, poor heat-resistant processability, etc., so as to highlight the style of cigarettes and improve the smoking quality. , the effect of high stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] In a 50ml round-bottomed flask, 8ml of anhydrous ether and 0.311ml (2.2mmol) of diisopropylamine were added, the solution was cooled to -60°C, and then BuLi (2.2mmol) of 2.5N n-hexane solution was added dropwise to the reaction system, The reaction system was raised to 0°C and stirred for 15min, then the ether solution of ambroxolide (500mg, 2.0mmol) was added dropwise at -60°C, the reaction system was continued to stir for 40min, and then 3-acetylpyridine (242.2mg, 2.0 mmol) was added dropwise. mmol) in ether was added dropwise to the reaction system. The reaction system was stirred at this temperature for 50 min, quenched with water, the organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, distilled under reduced pressure to remove the solvent, and the residue was separated by silica gel column chromatography to obtain CYL- 2-QX-2A (a mixture of two diastereomers CYL-2-QX-2A-a and CYL-2-QX-2A-b), 68.0% yield.
[004...
Embodiment 2
[0052] In a 50ml round-bottomed flask, 8ml of anhydrous ether and 0.311ml (2.2mmol) of diisopropylamine were added, the solution was cooled to -60°C, and then BuLi (2.2mmol) of 2.5N n-hexane solution was added dropwise to the reaction system, The reaction system was raised to 0 °C and stirred for 15 min, and then a solution of ambroxolide (500 mg, 2.0 mmol) in ether was added dropwise at -60 °C, the reaction system was continued to stir for 40 min, and then 2-acetylpyridine (242.2 mg, 2.0 mmol) was added dropwise. 2.0 mmol) ether solution was added dropwise to the reaction system. The reaction system was stirred at this temperature for 50 min, quenched with water, the organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, distilled under reduced pressure to remove the solvent, and the residue was separated by silica gel column chromatography to obtain CYL- 2-QX-2B, yield 63.0%.
[0053] Among them, the structure of CYL-2-QX-2B...
Embodiment 3
[0058] In a 50ml round-bottomed flask, 8ml of anhydrous ether and 0.311ml (2.2mmol) of diisopropylamine were added, the solution was cooled to -60°C, and then BuLi (2.2mmol) of 2.5N n-hexane solution was added dropwise to the reaction system, The reaction system was raised to 0°C and stirred for 15min, then the ether solution of ambroxolide (500mg, 2.0mmol) was added dropwise at -60°C, the reaction system was continued to stir for 40min, and then 4-acetylpyridine (242.2mg, 2.0 mmol) was added dropwise. mmol) in ether was added dropwise to the reaction system. The reaction system was stirred at this temperature for 50 min, quenched with water, the organic layer was separated, washed with saturated brine, dried over anhydrous sodium sulfate, filtered, distilled under reduced pressure to remove the solvent, and the residue was separated by silica gel column chromatography to obtain CYL-2- QX-2C, yield 61.0%.
[0059] ESI MS(positive ion mode)(rel.int.)m / z:372([M+H] + ,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com