Aromatic phenolic ester precursor-aroma compound as well as preparation method and application thereof
A technology for aromatic phenolic esters and compounds, which is applied in the direction of condensation preparation of carbonyl compounds, preparation of tobacco, essential oils/spice, etc., can solve the problems of affecting the quality stability, unstable content and high volatility of tobacco products, and achieves rich variety, widen Scope of application, effect of high volatility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0039] Add 15ml of dichloromethane and 0.8ml of triethylamine to 5mmol of vanillin, and stir in an ice bath for 10 minutes; dissolve 5mmol of acid chloride in 5ml of dichloromethane, slowly drop into the vanillin solution, and stir at 25°C for 8h. Add 200ml of dichloromethane to the reaction product, wash with 1M dilute hydrochloric acid three times, 50ml each time, and then wash once with 50ml of saturated brine, concentrate in vacuo to remove solvent, and use petroleum ether: ethyl acetate = 20:1-15:1 column chromatography The product LSD8 was isolated with a yield of 76%.
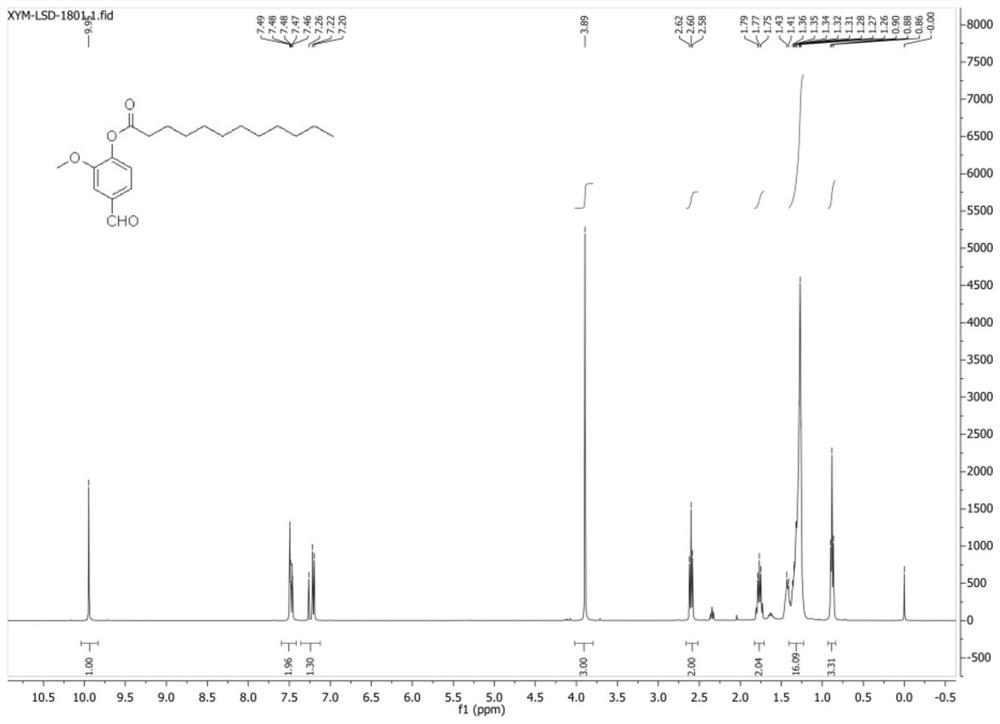
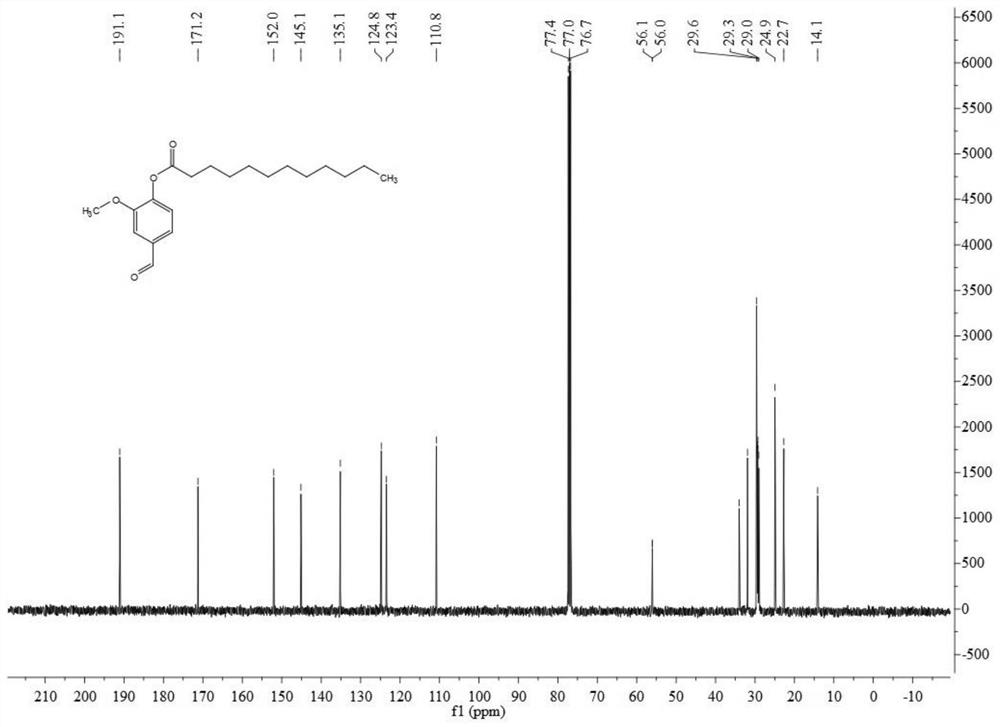
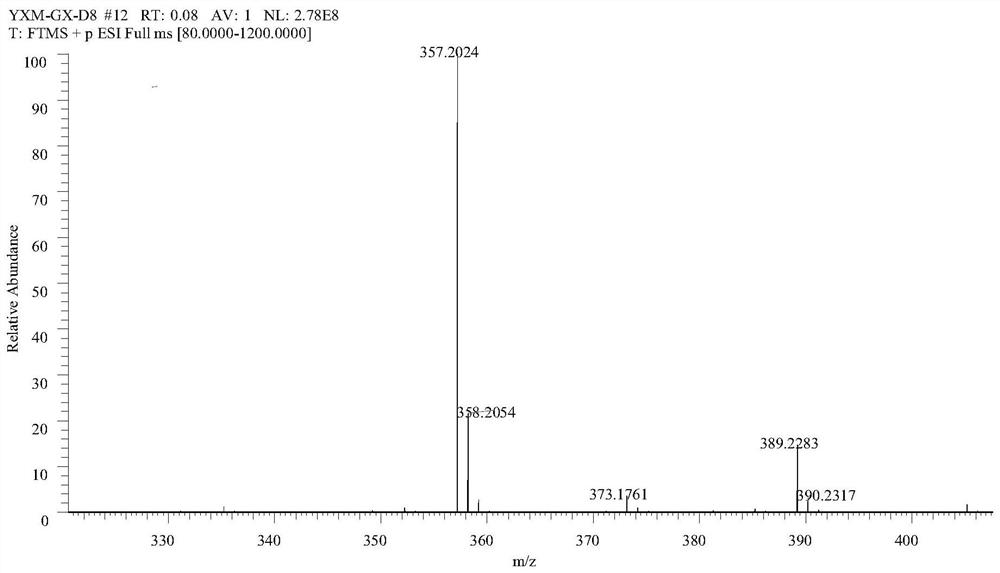
[0040] Test results such as Figure 1-3 As shown, wherein the structural representation is as follows:
[0041] LSD-08: 1HNMR (400MHz, CDCl3): δ, ppm 0.88(t, 3H), 1.26~1.43(m, 16H), 1.73~1.80(m, 2H), 2.6(t, 2H), 3.89(s, 3H),7.21(d,1H),7.46~7.49(m,2H),9.95(s,1H); 13CNMR(100MHz,CDCl3):δ,ppm 191.1,171.2,152.0,145.1,135.1,124.8,123.4, 110.8, 56.0, 34.0, 31.9, 29.6, 29.5, 29.3, 29.0, 24.9, 22.7; ESI-MS (m / ...
Embodiment 2
[0043]Add 15ml of dichloromethane and 0.8ml of triethylamine to 5mmol of ethyl vanillin, and stir in an ice bath for 10 minutes; dissolve 5mmol of tetradecanoyl chloride in 5ml of dichloromethane, and slowly add it dropwise to the vanillin solution at 25°C Under stirring for 8h. Add 200ml of dichloromethane to the reaction product, wash with 1M dilute hydrochloric acid three times, 50ml each time, and then wash once with 50ml of saturated brine, concentrate in vacuo to remove the solvent, and use petroleum ether: ethyl acetate = 20:1-15:1 column chromatography The product LSD13 was isolated in 89% yield.
[0044] Test results such as Figure 4-6 As shown, wherein the structural representation is as follows:
[0045] LSD-13: 1HNMR (400MHz, CDCl3): δ, ppm 0.88(t, 3H), 1.26~1.36(m, 18H), 1.40~1.46(m, 5H), 1.74~1.81(m, 2H), 2.59( t, 2H), 4.13 (dd, 2H), 7.20 (d, 1H), 7.44~7.47 (m, 2H), 9.93 (s, 1H); 13CNMR (100MHz, CDCl3): δ, ppm 191.1, 171.2, 151.4 ,145.3,135.1,124.6,123.4,111...
Embodiment 3
[0047] Add 15ml of dichloromethane and 0.8ml of triethylamine to 5mmol of maltol, and stir in an ice bath for 10 minutes; dissolve 5mmol of oleoyl chloride in 5ml of dichloromethane, slowly drop into the vanillin solution, and stir at 25°C for 8h. Add 200ml of dichloromethane to the reaction product, wash with 1M dilute hydrochloric acid three times, 50ml each time, and then wash once with 50ml of saturated brine, concentrate in vacuo to remove the solvent, and use petroleum ether: ethyl acetate = 20:1-15:1 column chromatography The product LSD18 was isolated with a yield of 82%.
[0048] Test results such as Figure 7-9 As shown, wherein the structural representation is as follows:
[0049] LSD-18: 1HNMR (400MHz, CDCl3): δ, ppm 0.88(t, 3H), 1.23~1.43(m, 20H), 1.72~1.77(m, 2H), 1.96~2.06(m, 5H), 2.25( s,3H),2.60(t,2H),4.12(q,1H),5.33~5.38(m,2H),6.39(d,2H),7.67(d,1H); 13CNMR(100MHz,CDCl3):δ ESI- MS(m / z):399.2492[M+Na]+
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com