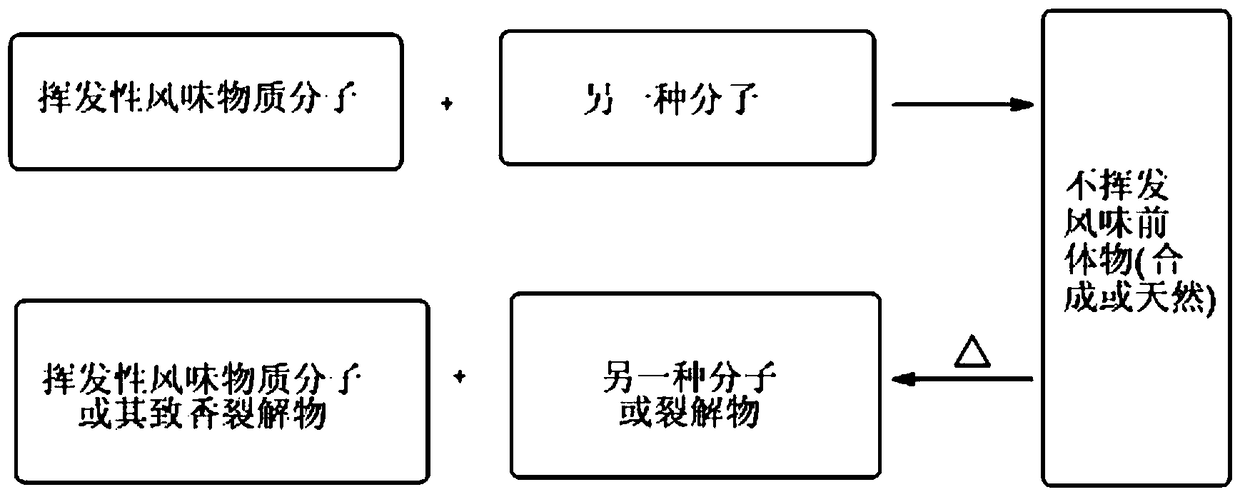
Latent aroma compound with sweet and roasted aroma and its preparation method and application
A compound and latent aroma technology, applied in the field of tobacco flavor, can solve the problems of poor use of tobacco formula, unstable quality of cigarettes, poor heat-resistant processability, etc., and achieve the effect of rich variety, small molecular weight, and heavy smell
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
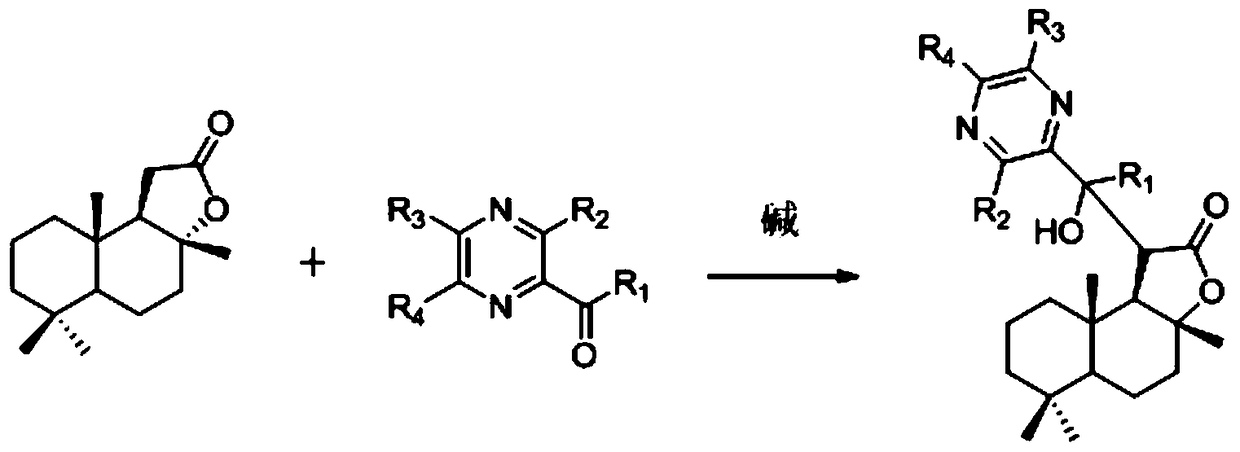
[0042] Add 8ml of anhydrous diethyl ether and 0.311ml (2.2mmol) of diisopropylamine in a 50ml round bottom flask, cool the solution to -60°C, then drop BuLi (2.2mmol) in 2.5N n-hexane solution into the upper reaction system, The reaction system was raised to 0°C and stirred for 15 minutes, then a diethyl ether solution of ambroxolide (500 mg, 2.0 mmol) was added dropwise at -60° C, and the reaction system was stirred for 40 minutes, then 2-acetylpyrazine (244.2 mg, 2.0 mmol) of ether solution was added dropwise to the reaction system. Stir the reaction system at this temperature for 50 min, quench the reaction with water, separate the organic layer, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and separate the residue by silica gel column chromatography to obtain CYL-2 -QX-4A (mixture of two diastereoisomers CYL-2-QX-4A-a and CYL-2-QX-4A-b), yield 61.0%, structure of its diastereomers Characterized as fo...
Embodiment 2
[0052] Add 8ml of anhydrous diethyl ether and 0.311ml (2.2mmol) of diisopropylamine in a 50ml round bottom flask, cool the solution to -60°C, then drop BuLi (2.2mmol) in 2.5N n-hexane solution into the upper reaction system, The reaction system was raised to 0°C and stirred for 15 minutes, then a diethyl ether solution of ambroxolide (500 mg, 2.0 mmol) was added dropwise at -60° C, and the reaction system was stirred for 40 minutes, and then 3-methyl-2-acetylpyrrolide was added A diethyl ether solution of oxazine (273.3mg, 2.0mmol) was added dropwise to the reaction system. Stir the reaction system at this temperature for 50 min, quench the reaction with water, separate the organic layer, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and separate the residue by silica gel column chromatography to obtain CYL-2- QX-4B, yield 59.0%.
[0053] Structural characterization of CYL-2-QX-4B:
[0054] 1 HNMR (CDCl...
Embodiment 3
[0058] Add 8ml of anhydrous diethyl ether and 0.311ml (2.2mmol) of diisopropylamine in a 50ml round bottom flask, cool the solution to -60°C, then drop BuLi (2.2mmol) in 2.5N n-hexane solution into the upper reaction system, The reaction system was raised to 0°C and stirred for 15 minutes, then a diethyl ether solution of norambrolide (500mg, 2.0mmol) was added dropwise at -60°C, the reaction system was stirred for 40 minutes, and then 5-methoxy-2-acetyl Ether solution of pyrazine (304.3 mg, 2.0 mmol) was added dropwise to the above reaction system. Stir the reaction system at this temperature for 50 min, quench the reaction with water, separate the organic layer, wash with saturated brine, dry over anhydrous sodium sulfate, filter, distill off the solvent under reduced pressure, and separate the residue by silica gel column chromatography to obtain CYL-2- QX-4C, yield 55.0%.
[0059] ESI-MS(positive ion mode)(rel.int.)m / z:403([M+H] + ,100).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com