Menthol formate precursor-aroma compound as well as preparation method and application thereof
A technology of alcohol formate and compounds, which is applied in the field of menthyl formate latent aroma compounds, can solve the problems of mint flavor loss, poor stability, and high volatility, and achieve the effects of high volatility, stable properties, and increased types
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
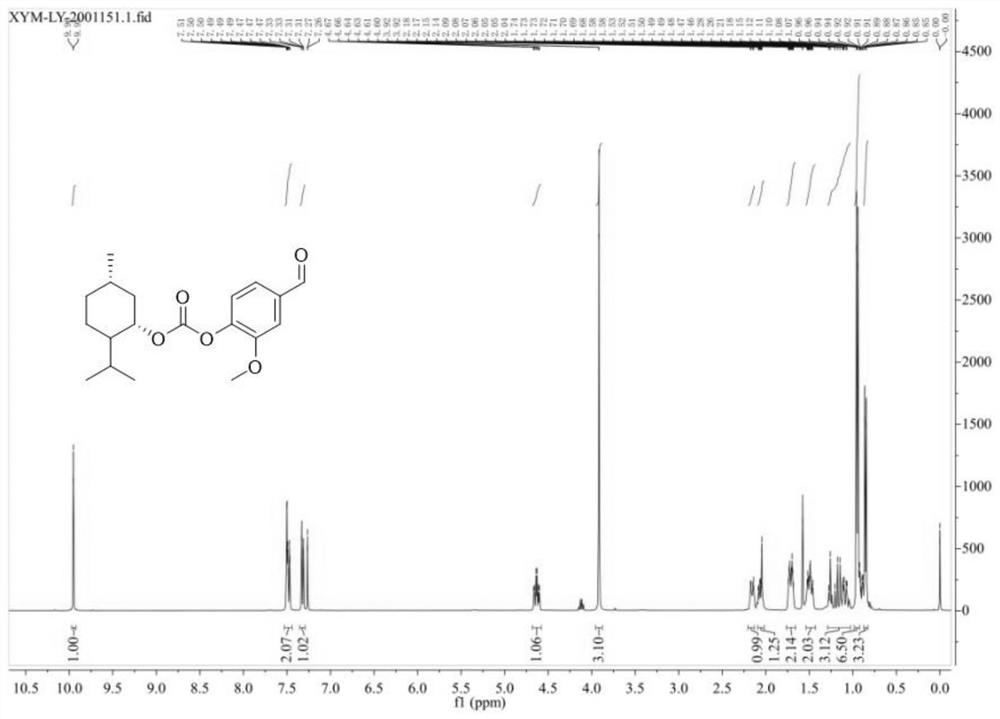
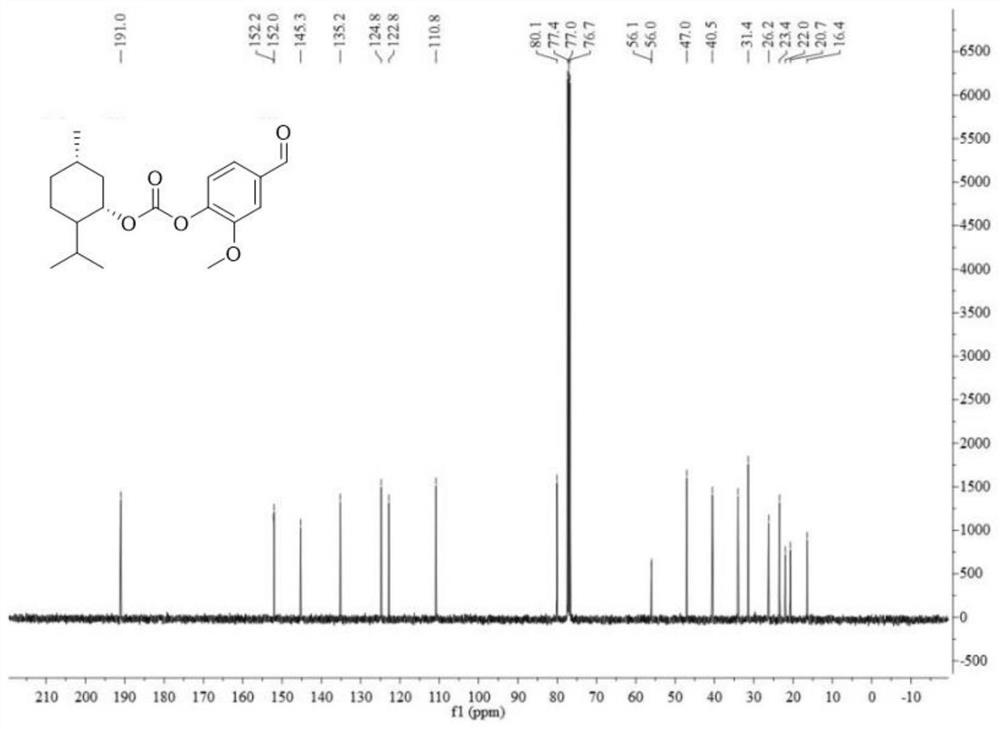
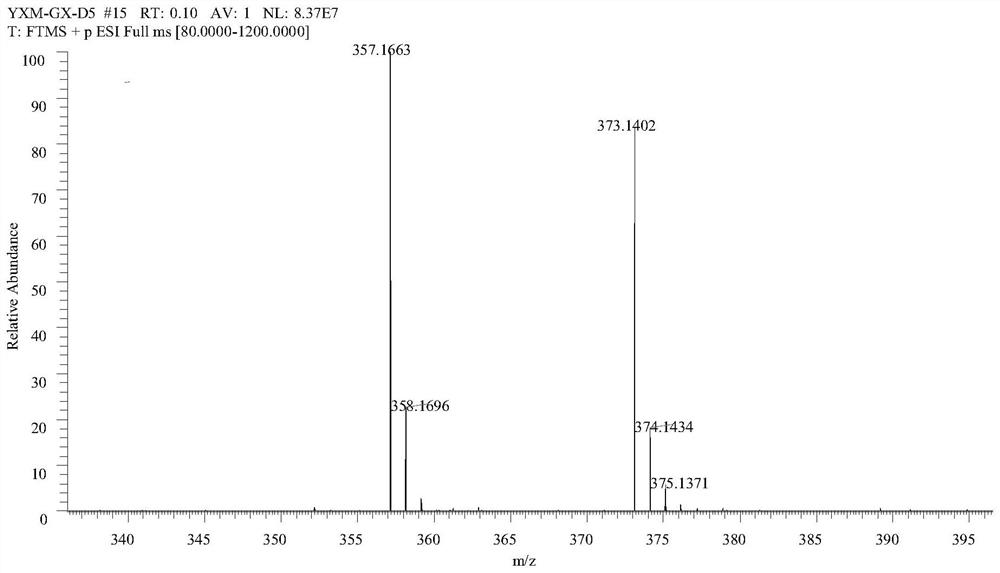
[0037] Methol (0.75 g, 4.81 mmol) was added (no water) dichloromethane (10 mL) to dissolve, and three light gas (0.58 g, 1.77 mmol, water-water dichloromethane dissolved) was stirred, and pyridine (0.4 ml, 4.91) mmol). 50-60 ° C was stirred for 5 h, and the reduced pressure was concentrated (about 1.75 g, about 4.8 mmol) was added, and dichloromethane was dissolved, and the fragrant of 4 mmol was added, and the catalytic amount of triethylamine was added, stirred even about 20 min. After slow adding to the above solution, the reaction overnight, the TLC point plate was almost reacted. The reaction system was dried, the water and ethyl acetate were added, and the organic phase was retained, and the water was washed twice. The product LY1 was isolated by petroleum ether: ethyl acetate = 20: 1-15: 1 column chromatography obtained by 78% yield.
[0038] Test results Figure 1-3 As shown, the structure characterization is as follows:
[0039] 1 HNMR (400MHz, CDCL 3 : δ, PPM 0.85 (D, 3H)...
Embodiment 2
[0041]Methol (0.75 g, 4.81 mmol) was added (no water) dichloromethane (10 mL) to dissolve, and three light gas (0.58 g, 1.77 mmol, water-water dichloromethane dissolved) was stirred, and pyridine (0.4 ml, 4.91) mmol). 50-60 ° C was stirred for 5 h, concentrated under reduced pressure (lysolated solid 1.75 g, about 4.8 mmol), dissolved in dichloromethane, and additionally ethylcetin 4 mmol, add catalytic amount of triethylamine, stir well After about 20 min, slowly added to the above solution, and the reaction was overnight. The reaction system was dried, the water and ethyl acetate were added, and the organic phase was retained, and the water was washed twice. The product Ly2 was isolated by petroleum ether: ethyl acetate = 20: 1-15: 1 column chromatography was isolated from 76% yield.
[0042] Test results Figure 4-6 As shown, the structure characterization is as follows:
[0043] 1 HNMR (400MHz, CDCL 3 : δ, PPM 0.85 (D, 3H), 0.95 (D, 6H), 1.03 ~ 1.46 (m, 3H), 1.42 ~ 1.46 (M, 3H)...
Embodiment 3
[0045] Methol (0.75 g, 4.81 mmol) was added (no water) dichloromethane (10 mL) to dissolve, and three light gas (0.58 g, 1.77 mmol, water-water dichloromethane dissolved) was stirred, and pyridine (0.4 ml, 4.91) mmol). 50-60 ° C for 5 h, concentrated under reduced pressure (about 1.75 g, about 4.8 mmol), and dichloromethane dissolved, and maltol 4mmol, add catalytic amount of triethylamine, stir evenly about 20 minutes. Added to the above solution, the reaction overnight. The reaction system was dried, the water and ethyl acetate were added, and the organic phase was retained, and the water was washed twice. The product LY4 was isolated by petroleum ether: ethyl acetate = 20: 1-15: 1 column chromatography was obtained from the product LY4, and the yield was 72%.
[0046] Test results Figure 7-9 As shown, the structure characterization is as follows:
[0047] 1 HNMR (400MHz, CDCL 3 : δ, PPM 0.85 (D, 3H), 0.94 (D, 6H), 1.02 ~ 1.54 (m, 2H), 1.68 ~ 1.73 (m, 2H), 2.03 ~ 2.10 ( M, 1H), ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com