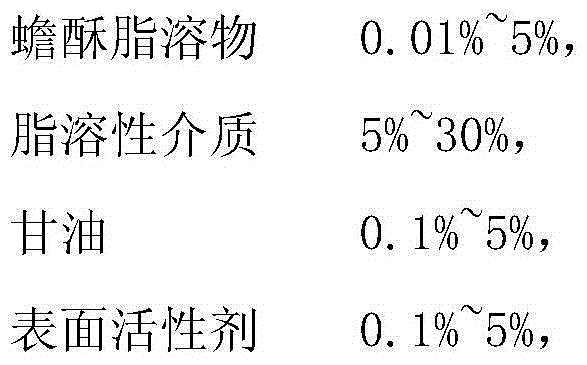
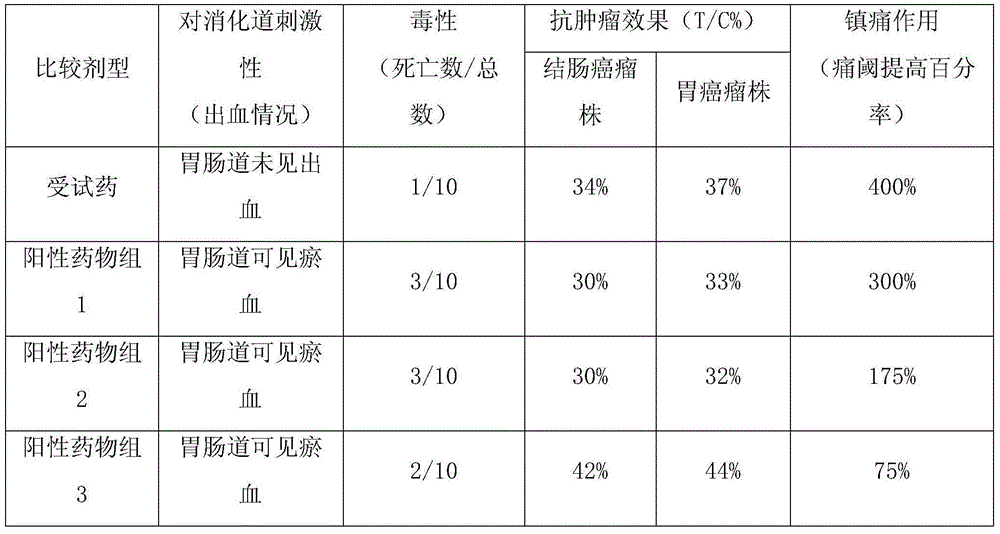
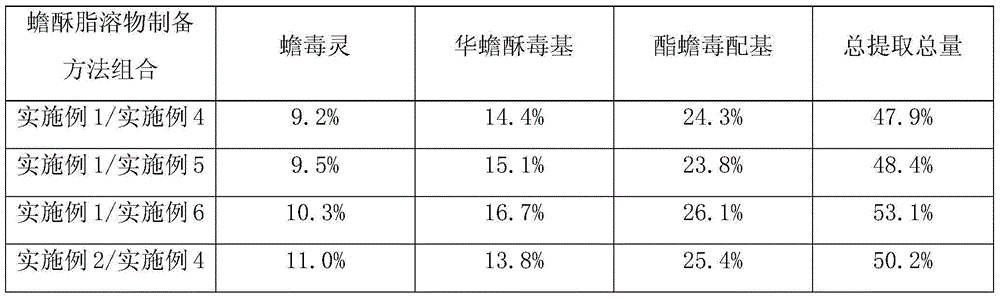
Medicine containing toad venom lipid-soluble substances and preparation method thereof
A technology of toad venom fat-soluble matter and toad venom, which is applied in the field of medicine, can solve the problems of high cost, poor safety, and side effects, and achieve the effects of reducing toxicity, improving stability, and reducing direct stimulation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Extraction of Toad Sutra
[0060] Crush the toad venom medicinal material and pass through a 200-mesh sieve to obtain the fine powder of the toad venom medicinal material. Weigh the fine powder, add 5 times the amount of absolute ethanol, heat and reflux in a water bath at 60°C for 1 hour, let cool to room temperature, suction filter, and evaporate the filtrate to dryness , and the dried filtrate was dried in a vacuum oven (60° C.) for 24 hours to obtain the extract of toad venom.
Embodiment 2
[0062] Extraction of Toad Sutra
[0063] Crush the toad venom medicinal material and pass through a 200-mesh sieve to obtain the fine powder of the toad venom medicinal material. Weigh the fine powder, add 15 times the amount of absolute ethanol, heat and reflux in a water bath at 60°C for 1 hour, let cool to room temperature, suction filter, and evaporate the filtrate to dryness , and the dried filtrate was dried in a vacuum oven (60° C.) for 24 hours to obtain the extract of toad venom.
Embodiment 3
[0065] Extraction of Toad Sutra
[0066] Crush the toad venom medicinal material and pass through a 200-mesh sieve to obtain the fine powder of the toad venom medicinal material. Weigh the fine powder, add 10 times the amount of absolute ethanol, heat and reflux in a water bath at 60°C for 1 hour, let cool to room temperature, suction filter, and evaporate the filtrate to dryness , and the dried filtrate was dried in a vacuum oven (60° C.) for 24 hours to obtain the extract of toad venom.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com