Nitrogen-containing sulfur substituent naphthalimide compound, preparation method and applications thereof
A technology of naphthalene imide and compound, applied in the field of organic synthesis, can solve the problems of limited clinical activity and unsatisfactory performance, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
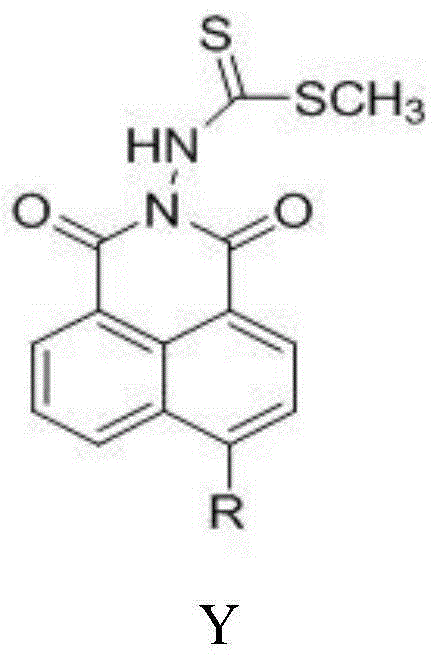
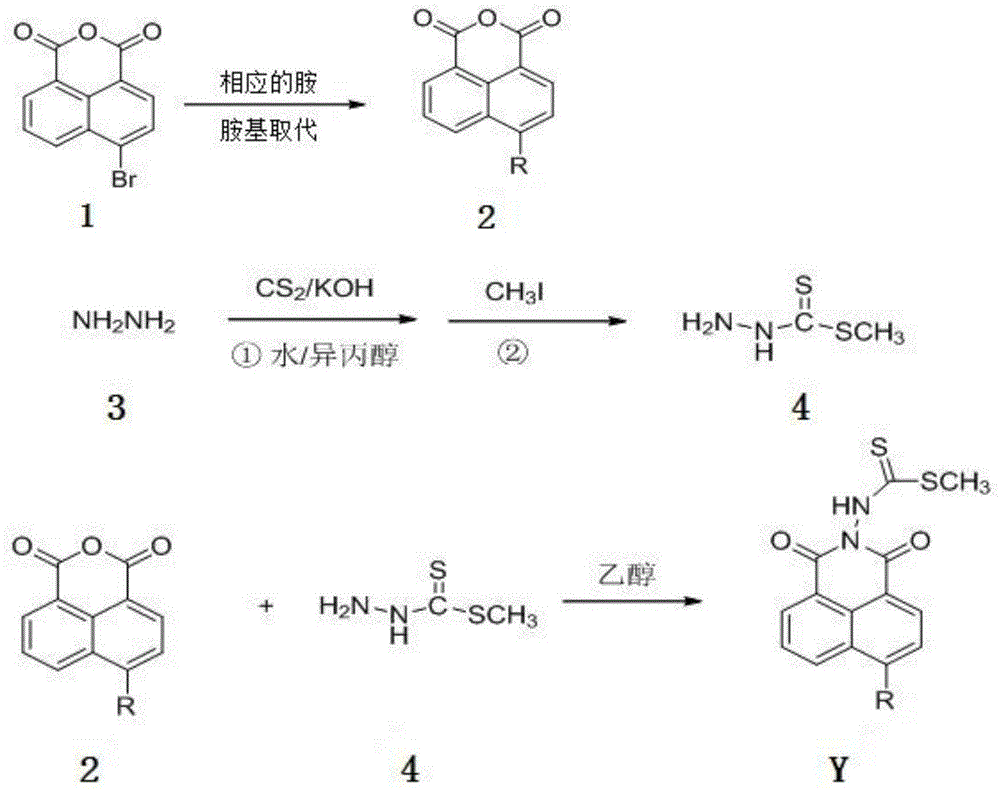
[0028] Synthesis of N-(N'-methyl dithioformate amino)-6-piperidinyl-1,8-naphthalimide (compound Y1):
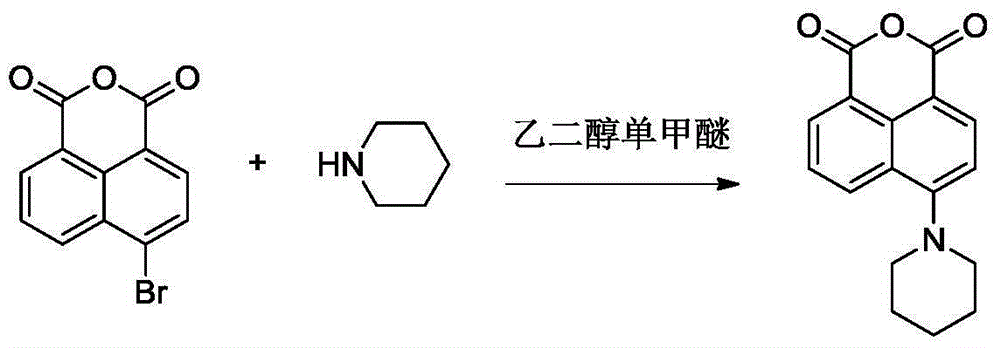
[0029] (1) Synthesis of 4-piperidinyl-1.8-naphthalene anhydride
[0030]
[0031] Add 5g (18.1mmol) of 4-bromo-1,8 naphthalene anhydride to a 100mL two-necked flask, add 40mL ethylene glycol monomethyl ether and stir to dissolve, add 2mL (20.2mmol) piperidine to the reaction system, heat to reflux, and magnetically After stirring for 4h, the reaction was stopped, cooled at room temperature, cold water was added to the reaction solution, a yellow precipitate was precipitated, filtered and dried, purified by silica gel column chromatography (eluent was CH 2 Cl 2 ) to obtain 4.77 g of yellow solid, yield: 93.6%.
[0032] (2) Synthesis of methyl dithiocarbazinate (compound 4)
[0033]
[0034] Add 6.6g (0.12mol) of potassium hydroxide, 8.0mL of water and 6.7mL of isopropanol into a 50mL three-neck flask, stir to dissolve, and slowly add 5.7mL (0.12mol) of hydrazine hydra...
Embodiment 2
[0042] Determination of growth inhibition activity of tumor cells and normal cells in vitro:
[0043] The in vitro cell growth inhibitory activity of Hela cervical cancer cells, MCF-7 breast cancer cells and HL7702 human normal liver cells was determined by tetrazolium salt (microculturetetrozolium, MTT) reduction method.
[0044] The concrete operation of tetrazolium salt (MTT) reduction method is:
[0045] (1) Inoculating cells and culturing cells: When the cells are in the logarithmic growth phase, digest the adherent cells with trypsin, collect them in serum-containing medium, and dilute the cell suspension to a concentration of about 4×10 5 ~6×10 5 individual / mL. Inoculate the above culture medium into a sterile 96-well plate, add 100 μL of cell suspension to each well (about 5000 cells per well), and add 200 μL of PBS buffer to each well at the outermost periphery to provide sufficient water environment for the cell growth ring. Place the inoculated culture plate at 3...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com