Fully human monoclonal antibody against CD20 and its application
A monoclonal antibody, fully human technology, applied in the direction of antibodies, applications, anti-animal/human immunoglobulin, etc., can solve the problems of continuous drug use, long-term drug use, multiple infusion reactions, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of hybridoma and carrier
[0064] 1. Antigen and lymphocyte preparation and immunization
[0065] 1.1 Antigen preparation:
[0066] 1.1.1 CD20 protein
[0067] Human CD20 (Novusbio) antigen was purchased, diluted with PBS, and filtered with a 0.22 μm needle sterile filter.
[0068] 1.1.2 Preparation of 293F cells expressing CD20 membrane protein
[0069] Purchase CD20 cDNA (Origene, SC101205), and design and synthesize PCR amplification primers:
[0070] CD20 forward primer: 5'-TCAGGAGTTTTGAGAGCAAAATG-3'
[0071] CD20 reverse primer: 5'-AACAGAAGAATCACTTAAGGAG-3'
[0072] Then use the cDNA of CD20 as a template to amplify and purify, and clone into the pCR3.1 vector (Invitrogen, K300001). TMAfter 48 hours, 293-F cells (Invitrogen, R790-07) were cultured with 1 mg / ml G418 (Invitrogen, 10131035) for 2 weeks. Stable G418-resistant clones were screened out and frozen.
[0073] 1.1.3 Extracted CD20 membrane protein
[0074] CD20 membran...
Embodiment 2
[0105] Example 2: Preparation and Identification of Monoclonal Antibody
[0106] 1. Preparation of antibodies by in vitro culture method
[0107] The 1.1.81, 1.4.68, 1.6.14, 1.36.2, 1.72.108, 1.89.45, 1.105.3, 1.134.42, 1.146.78, 1.176.109 cells, gradually reduce serum to serum-free or serum-free medium containing only a small amount of serum (Invitrogen, 12338-026), put in 225cm 2 Square bottle, according to inoculation 1×10 5 cells / ml, inoculate 100ml, and inoculate 4 bottles of each strain of cells.
[0108] 2. Antibody purification
[0109] Take out at 225cm 2 The cell supernatant of the square flask cultured for about 10 days was centrifuged to remove cell debris, and the antibody was purified by rProteinA affinity chromatography. Loading: the cell culture supernatant at pH 7.4 was filtered through a 0.22um filter membrane and loaded directly; washing: washing with PBS at pH 7.4, 10 times the column bed volume; elution: glycine solution at pH 3.0, each section C...
Embodiment 3
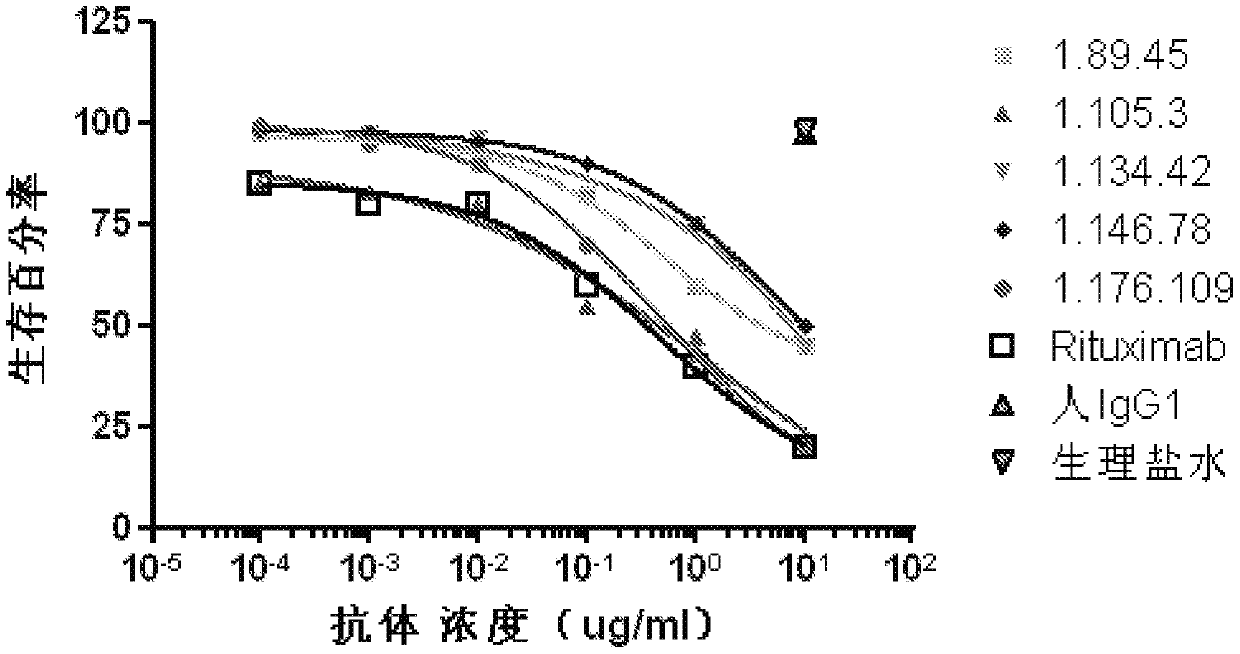
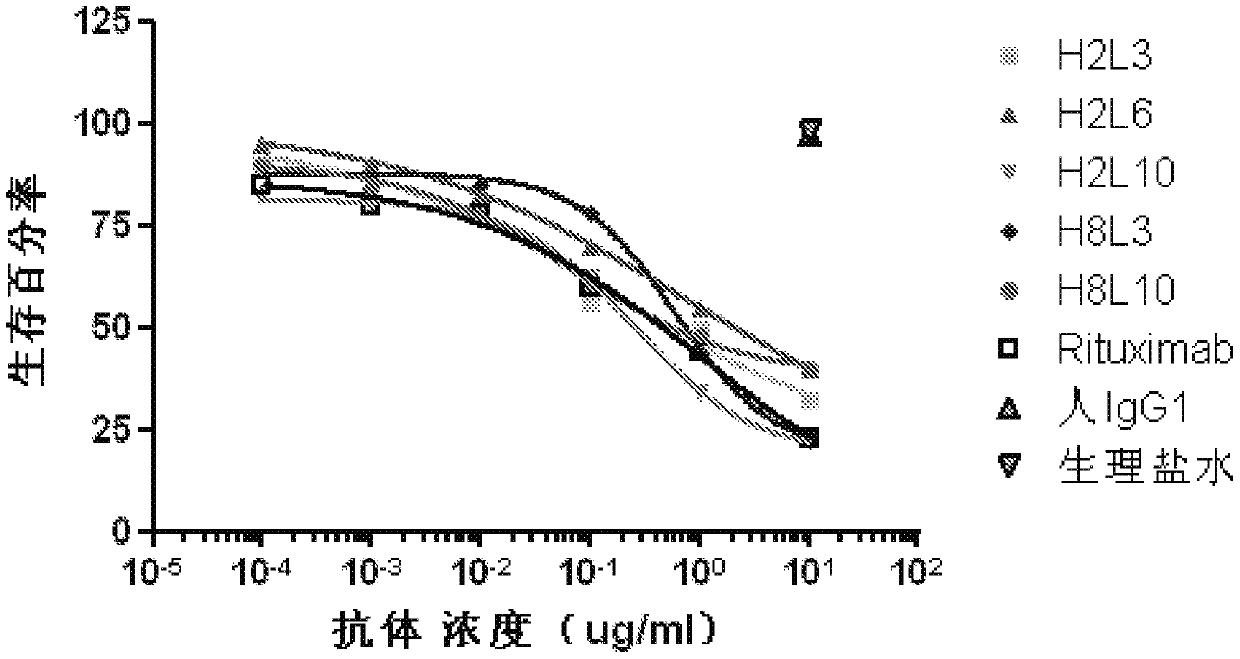
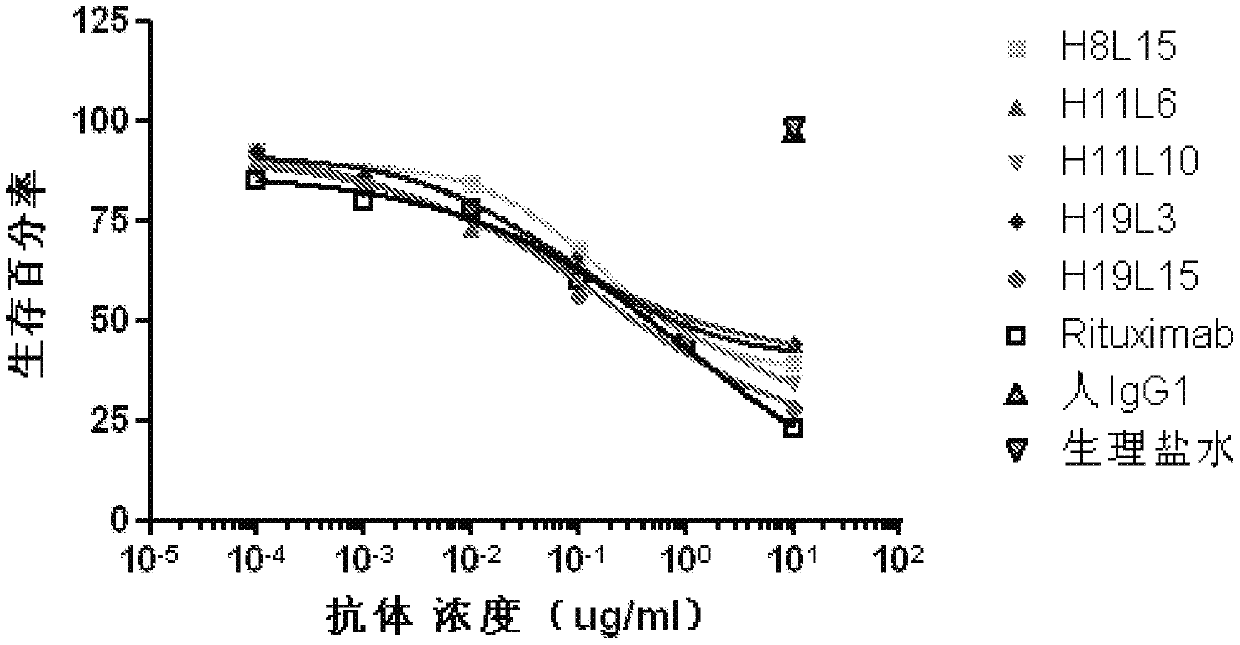
[0110] Example 3: Complement-dependent cytotoxicity assay (CDC)
[0111] Ramos, Raji and Daudi cells were purchased from ATCC in DMEM buffered with 10% FBS, 1% sodium pyruvate and 1% HEPES. CellTiterGlo kit was purchased from Promega (Madison, WI). Normal human serum was obtained from whole blood of healthy blood donors.
[0112] Press 1×10 5 cells / well, spread Ramos, Raji and Daudi cells into 96-well plates. At 37°C, 5% CO 2 The cells in the cell incubator were incubated with different concentrations of the antibody to be tested for 10 minutes, wherein the antibody to be tested was the cell supernatant antibody obtained in Example 2 or the affinity matured antibody obtained in Example 6, the positive control was Rituxan, and the negative The control was purified human IgG (purified from healthy human serum). Then normal human serum was added to make the final concentration in the culture solution 10%. At 37°C, 5% CO 2 In a cell culture incubator, cells, different conce...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com