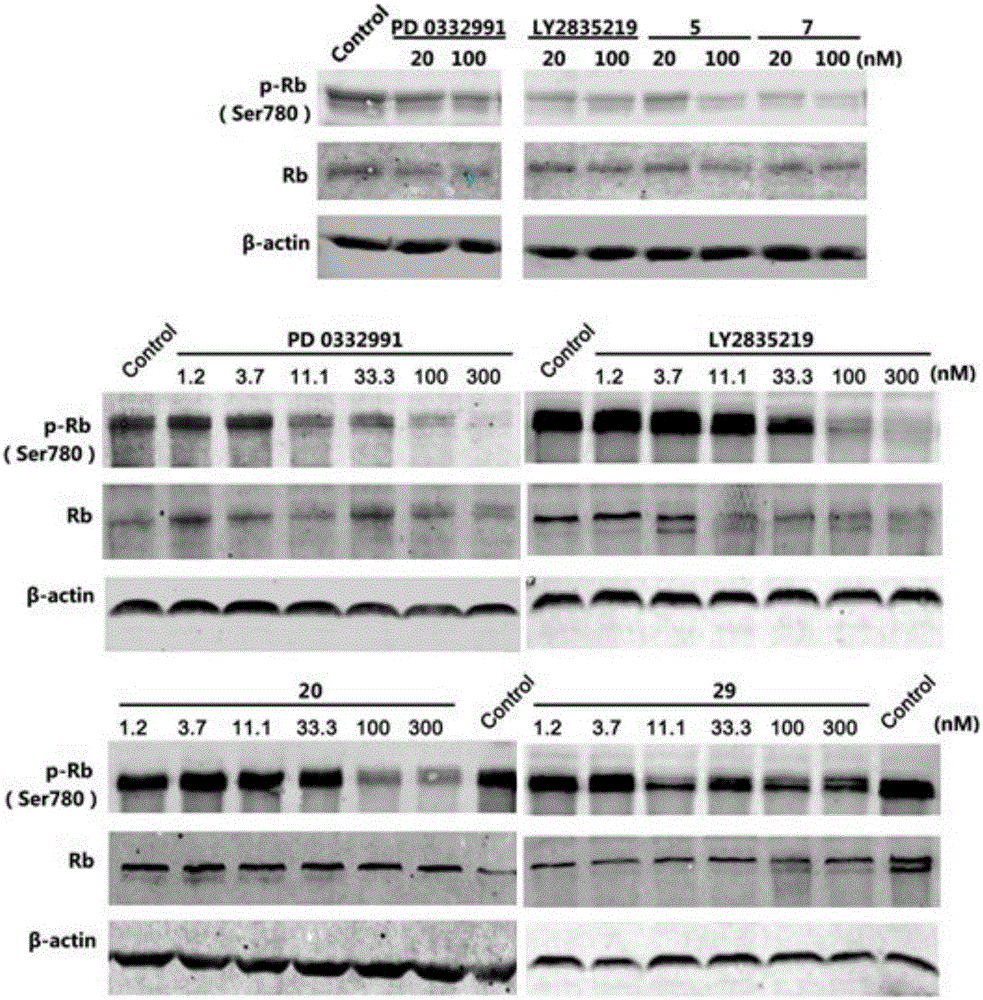
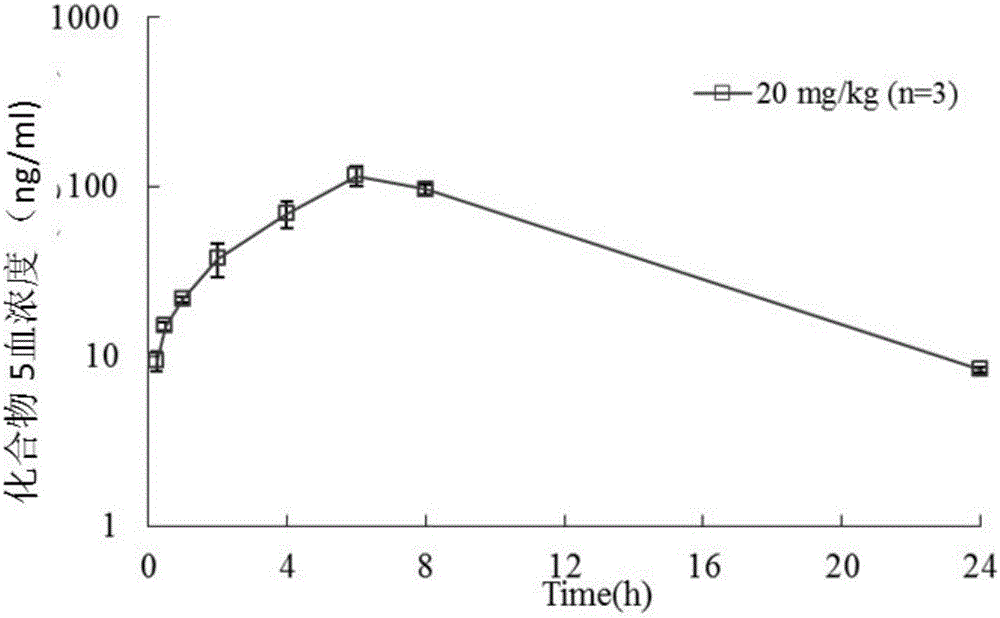
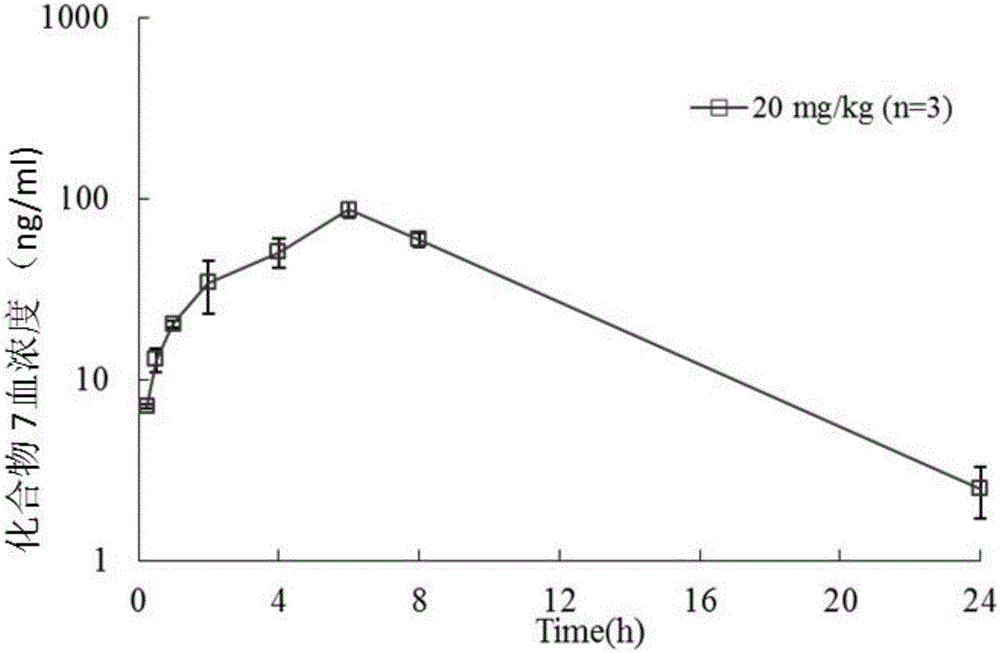
Pyridylpyrimidyl amine compounds or pyridylpyridyl amine compounds and application thereof
A technology of pyridine pyrimidine amine and pyridine pyridine amine, applied in the field of medicinal chemistry, can solve the problems of termination of clinical research, toxic and side effects, lack of selectivity of CDK subtype inhibition, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0107] Example 1: N 2 -(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-yl)-N 5 -(piperidine-4-methyl)pyridine-2,5-diamine (N 2 -(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzo[d]imidazol-6-yl)pyrimidin-2-yl)-N 5 -(piperidin-4-ylmethyl)pyridine-2,5-diamine) (compound 1) preparation
[0108] Step 1a: Preparation of N-isopropylacetamide (N-Isopropyl-acetamide) (compound 102-1)
[0109] Isopropylamine (10 g, 0.169 mol, 1.0 eq) and triethylamine (23.58 mL, 0.338 mol, 2.0 eq) were premixed in 100 mL of dichloromethane. Cool to 0°C, slowly add acetic anhydride (16.15 ml, 0.338 mol, 2.0 equiv) dropwise to the reaction solution, and react at room temperature overnight. Concentrate under reduced pressure, extract the concentrate with ethyl acetate, and dry the organic phase over anhydrous sodium sulfate. The organic phase was concentrated under reduced pressure to obtain compound N-isopropylacetamide (15.05 g, yield: 88%). 1 HNMR (500MHz, CDCl ...
Embodiment 2
[0128] Example 2: 2-[4-({6-[5-fluoro-4-(7-fluoro-2-methyl-3-propyl-3H-benzimidazol-5-yl)-pyrimidine-2- Amino]-pyridin-3-ylamino}-methyl)-piperidin-1-yl]-ethanol (2-[4-({6-[5-Fluoro-4-(7-fluoro-2-methyl -3-propyl-3H-benzoimidazol-5-yl)-pyrimidin-2-ylamino]-pyridin-3-ylamino}-methyl)-piperidin-1-yl]-ethanol) (compound 2)
[0129] Step 2a: Preparation of N-n-propylacetamide (N-propylacetamide) (compound 102-2)
[0130] Dissolve n-propylamine (5 grams, 0.085 moles, 1.0 equivalents) and triethylamine (11.79 milliliters, 0.169 moles, 2.0 equivalents) in 100 milliliters of dichloromethane, cool to 0 degrees, and add acetic anhydride (8.67 grams , 0.085 mol, 1.0 equivalent). After reacting overnight at room temperature, the solvent was spin-dried, and the concentrate was purified by alumina column chromatography (eluent: ethyl acetate) to obtain N-n-propylacetamide (7.5 g, yield: 88%) as a colorless oily liquid. LCMS(ESI):m / z102[M+H] + .
[0131] Step 2b: N'-(4-bromo-2,6-difluoro...
Embodiment 3
[0142] Example 3: N 5 -(1-Benzylpiperidin-4-ylmethyl)-N 2 -[5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3H-benzimidazol-5-yl)-pyrimidin-2-yl]-pyridine-2,5-diamine (N 5 -(1-Benzyl-piperidin-4-ylmethyl)-N 2 -[5-fluoro-4-(7-fluoro-3-isopropyl-2-methyl-3H-benzoimidazol-5-yl)-pyrimidin-2-yl]-pyridine-2,5-diamine) (Compound 3) preparation
[0143] Compound 4-((tert-butoxycarbonyl)(6-(5-fluoro-4-(4-fluoro-1-isopropyl-2-methyl-1H-benzimidazol-6-yl)pyrimidine-2 -yl)aminopyridin-3-yl))aminomethylpiperidine-1-carboxylic acid tert-butyl ester (109-1) (217 mg, 0.313 mmol, 1.0 equiv) was dissolved in dichloromethane (50 mL) , slowly dropwise into methanolic hydrochloric acid solution (8 ml), and stirred for 4 hours. The reaction solution was directly concentrated in vacuo to obtain a brown solid. The obtained solid and potassium carbonate (56 mg, 0.406 mmol, 1.4 eq) were added to a reaction flask equipped with acetonitrile (30 mL), followed by benzyl chloride (31 mg, 0.244 mmol, 0.8 eq...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com