Application of CABYR-a/b to promotion of sensibility of cancer cells to VP16 and TRAIL
A tumor cell, VP16 technology, applied in the application field of sensitivity, can solve problems that have not yet been seen
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
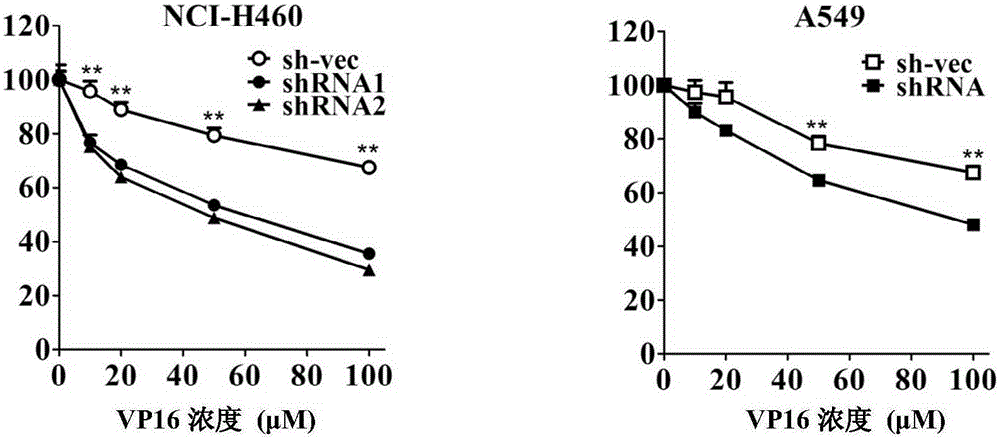
[0056] Example 1. Silencing CABYR-a / b can promote the sensitivity of lung cancer cells to the chemotherapeutic drug VP16
[0057] This example will involve CABYR-a / b protein and caspase3 protein. Wherein, the amino acid sequence of the CABYR-a protein (i.e., a subtype CABYR protein) is shown in sequence 4 in the sequence listing; the amino acid sequence of the CABYR-b protein (i.e., b subtype CABYR protein) is shown in sequence 5 in the sequence listing; The amino acid sequence of the caspase3 protein is shown in sequence 3 in the sequence listing; cleavedcaspase3 refers to a cleavage at the 175th aspartic acid of the protein shown in sequence 3.
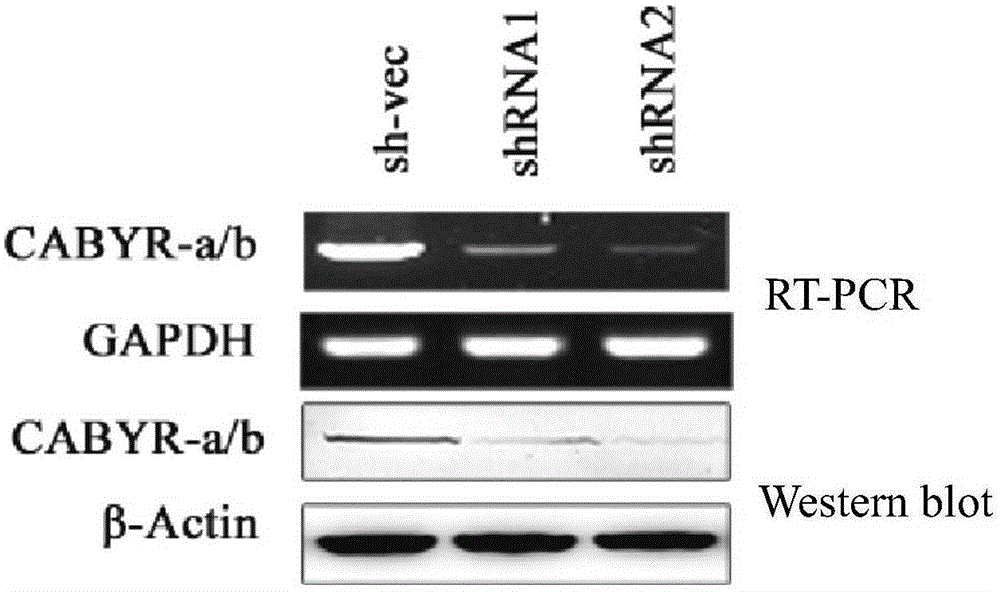
[0058] 1. Silencing CABYR-a / b in lung cancer cells
[0059] 1. Construct silencing vector
[0060] (1) Construction of silencing vector pGPH1 / Neo-CABYR-a / b-121
[0061] Artificially synthesize the following two single-stranded DNA molecules, CABYR-a / b-S1 and CABYR-a / b-A1, and anneal them into double strands. Then, the pGPH1 / Neo ...
Embodiment 2
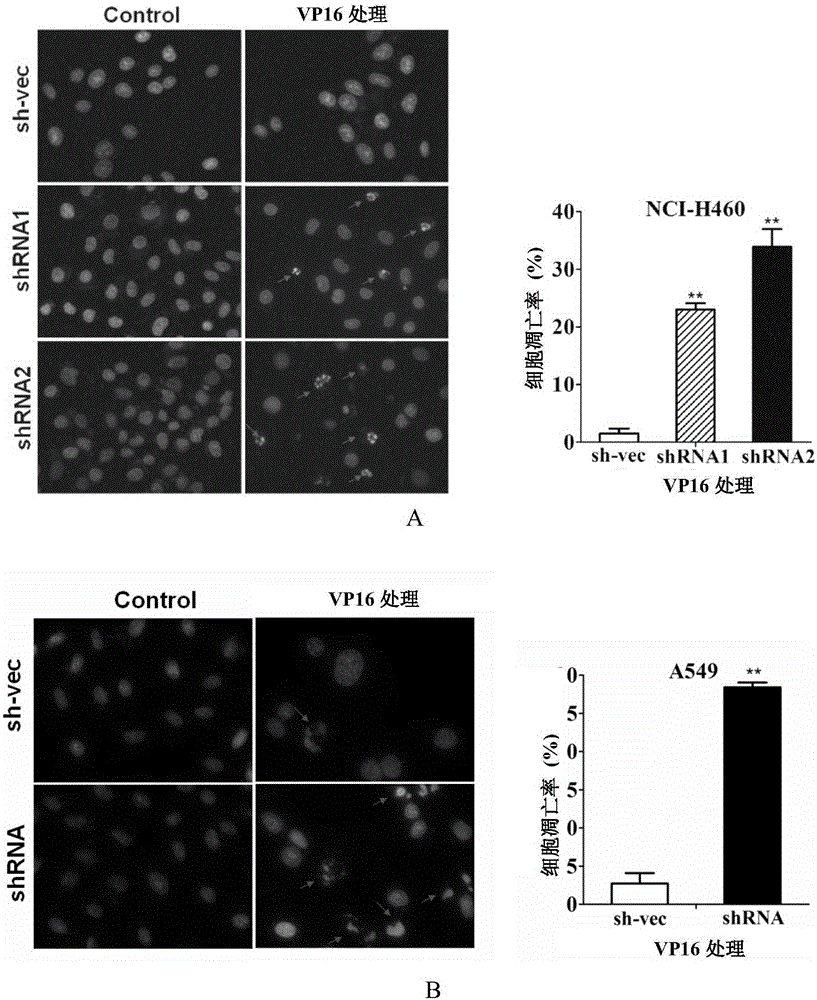
[0131] Example 2. Silencing CABYR-a / b can promote the sensitivity of lung cancer cells to TRAIL
[0132] In addition to CABYR-a / b protein, this embodiment also involves TRAIL protein and DR5 protein. Wherein, the amino acid sequence of TRAIL protein is shown in sequence 1 in the sequence listing; the amino acid sequence of DR5 protein is shown in sequence 2 in the sequence listing.
[0133] 1. Silencing of CABYR-a / b in NCI-H460 cells
[0134] Referring to Step 1 of Example 1, three kinds of monoclonal cells were finally obtained (into NCI-H460 cells, the silencing vectors pGPH1 / Neo-CABYR-a / b-121, pGPH1 / Neo-CABYR-a / b-303 and pGPH1 / Neo-CABYR-a / b-303 and nonsense interfering sequence).
[0135] 2. The cell survival rate of NCI-H460 cells stably silenced by CABYR-a / b was detected by MTT assay
[0136] Three kinds of monoclonal cells obtained in step 1 (into NCI-H460 cells were transferred into silencing vectors pGPH1 / Neo-CABYR-a / b-121, pGPH1 / Neo-CABYR-a / b-303 and nonsense inter...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com