Application of homoharringtonine-type compounds in antitumor drug preparation
A technology for homoharringtonine and harringtonine, which is applied in the fields of medicine and medicinal chemistry, and can solve the selection and application of clinical indications of hindering compounds, homoharringtonine and harringtonine target molecules and their target molecules. Molecular mechanism ominous and other issues
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
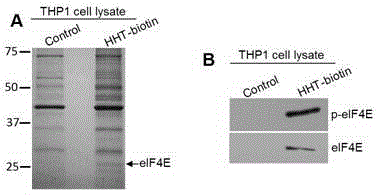
[0018] Example 1: Identification of the target molecule p-eIF4E for the antitumor effect of homoharringtonine
[0019](1) Biotin-labeled homoharringtonine and 1ml human leukemia THP-1 cell protein solution were incubated overnight at 4°C in a 1.5ml EP centrifuge tube to allow biotin-labeled homoharringtonine to interact with the protein Fully combined; (2) add an appropriate amount of avidin magnetic beads and incubate at room temperature for 1 hour, so that the protein specifically bound to homoharringtonine can be captured on the surface of avidin magnetic beads by biotin; (3) in the magnetic field Wash off the unbound protein with buffer on the rack; (4) add protein lysate and boil in a water bath for 5 minutes to lyse the protein specifically bound to homoharringtonine from the surface of the magnetic beads; (5) The lysed protein was separated by SDS-PAGE protein electrophoresis, and the protein band was stained with Coomassie brilliant blue; (6) The stained protein band...
Embodiment 2
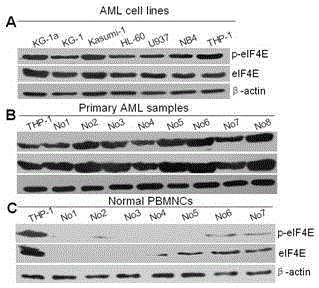
[0020] Example 2: Expression of phosphorylated eIF4E (p-eIF4E) in different tumor cell lines, primary tumor cell samples and normal cells
[0021] (1) Experimental materials
[0022] Leukemia cell lines: KG-1a, KG-1, Kasumi, HL-60, U937, NB4, THP-1. Primary leukemia cell samples were from different types of AML, and normal blood cell samples were from volunteers.
[0023] (2) The experimental method adopts conventional immunoblotting technique.
[0024] The cellular proteins of leukemia cells and normal blood cell samples were extracted according to conventional methods, separated by SDS-PAGE protein electrophoresis, and then transferred to NC membranes, and the experimental steps of primary antibody and secondary antibody incubation, color development, and exposure were performed according to conventional methods. β-actin was used as an internal reference. The result is as figure 2 It shows that p-eIF4E is highly expressed in leukemia cell lines (A) and some primary le...
Embodiment 3
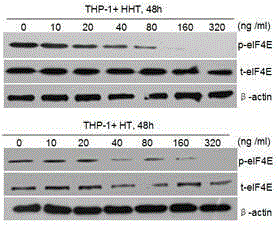
[0025] Example 3: Harringtonine and homoharringtonine inhibit the expression of p-eIF4E in human THP-1 leukemia cells
[0026] (1) Experimental materials
[0027] Leukemia cell line: human THP-1 leukemia cell line (acute myeloid leukemia-M5, AML-M5).
[0028] Reagents: harringtonine, homoharringtonine
[0029] (2) Experimental method
[0030] Well-grown leukemia cells were inoculated into the wells of a 6-well cell culture plate at a density of 1×10 6 / ml. The culture medium was 1640 cell culture medium containing 10% fetal bovine serum. Add different concentrations of harringtonine and homoharringtonine, mix well, and place in carbon dioxide (5% CO 2 ) cell incubator 37 oC Incubate for 48 hours. Then the cellular protein was extracted, and the expression level of p-eIF4E was detected by immunoblotting.
[0031] (3) Experimental results
[0032] See the experimental results image 3 . Harringtonine (HT) and homoharringtonine (HHT) down-regulated p-eIF4E levels in ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com