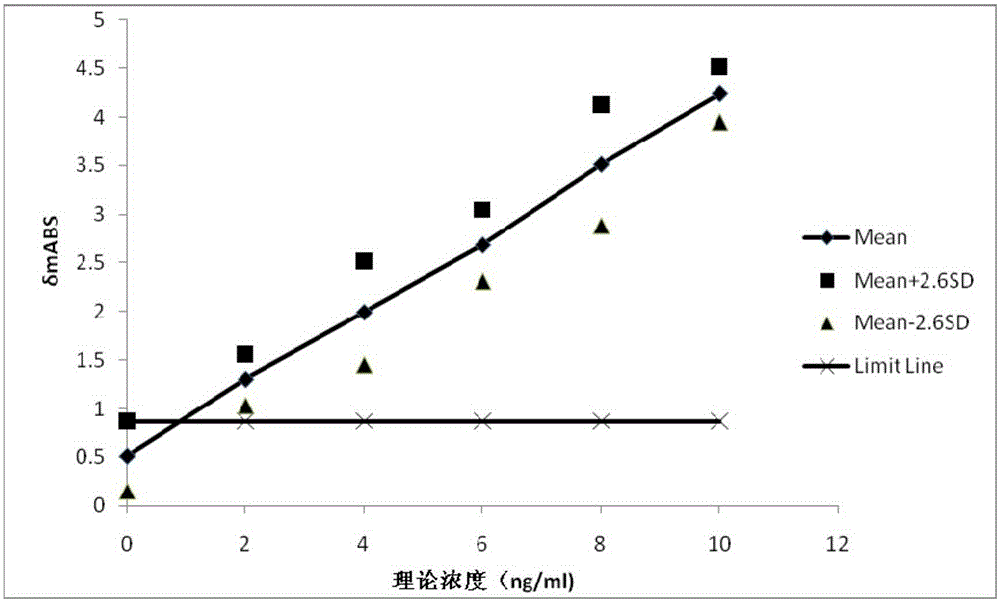
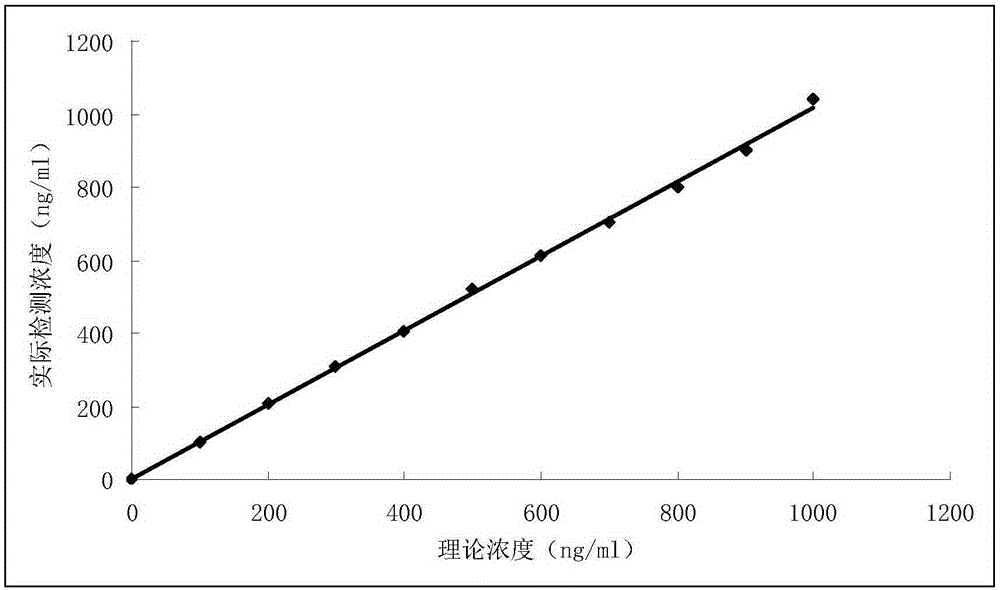
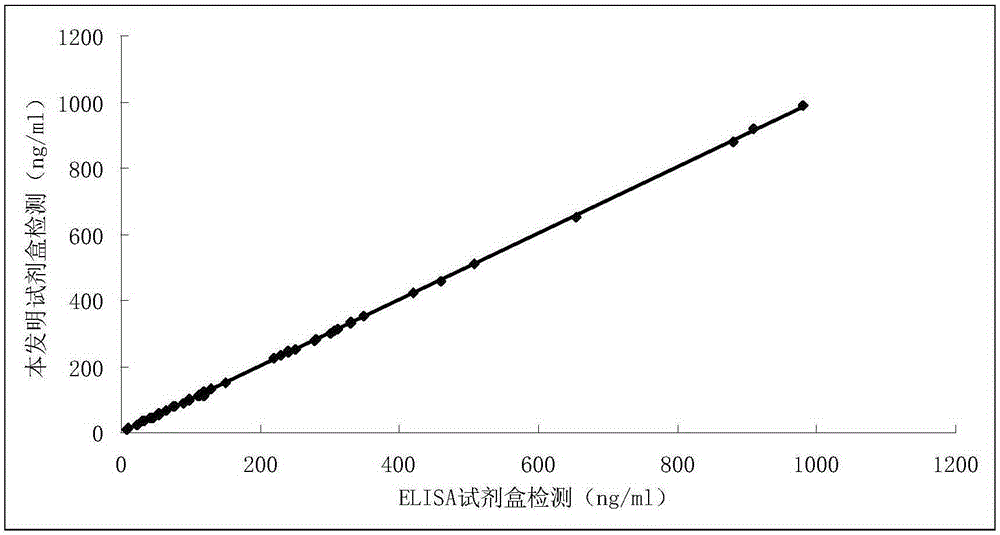
Diagnostic kit for in-vitro quantitative determination of GPI (Glucose-6-phosphateisomerase) with latex-enhanced immunoturbidimetry
An immunoturbidimetric and latex-enhanced technology, applied in disease diagnosis, measurement devices, instruments, etc., can solve the problems of cumbersome steps, unfavorable large-scale detection, time-consuming and labor-intensive, etc., and achieve the effect of easy and fast use.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Example 1: Preparation of goat anti-human GPI polyclonal antibody by affinity purification
[0032] Preparation of goat anti-human GPI polyclonal antibody:
[0033] Take healthy immunized animals and immunize with GPI antigen. For the first time, mix the antigen with BCG and Freund’s adjuvant, inject the animal’s cervical lymph nodes and subcutaneous muscle tissue, once every two weeks, and immunize 4-5 times in total. The amount of antigen depends on Animal weight gain and loss, blood collection two weeks after the last immunization, animal anti-human serum was preliminarily separated by secondary precipitation with saturated ammonium sulfate.
[0034] Purification of goat anti-human polyclonal antibody:
[0035] First, the separation column used for affinity purification was prepared: 10 mg of highly purified GPI antigen was immobilized on a HITRAPNHS-active HP column (from AmershamPharmaciaBiotech);
[0036] Next, sample loading and elution: Dilute the initially se...
Embodiment 2
[0038] Embodiment 2: Preparation of GPIR2 reagent
[0039] The process is divided into three steps: antibody cross-linking, latex washing, latex suspension
[0040] Antibody cross-linking: Dilute 0.5 mg of the antibody prepared above with 5 ml of 0.1M phosphate buffer (pH7.8), add surface-modified carboxyl groups, polystyrene latex solution (from JSR) with a diameter of 120 nm, and then add 5 mg of EDAC. React at 37°C for 6 hours, add 0.5ml of 0.1M glycine buffer (pH8.5) and stir for 1 hour to terminate the reaction.
[0041] Latex cleaning: Centrifuge to remove the supernatant to remove excess antibodies, cross-linking agents and by-products of cross-linking reactions, etc., and the bottom sediment is the coated latex. Wash 3 times with 20 ml of 50 mM glycine-NaOH buffer.
[0042] Latex suspension: Add 20ml of the same glycine-NaOH buffer solution to the above precipitation, ultrasonic treatment for 4min, and mix well to obtain a milky white latex suspension, which is the R...
Embodiment 3
[0044] Example 3: Preparation of GPIR1 reagent
[0045] Add 0.9% sodium chloride, 0.2% Tween 20, 5% PEG-6000, 0.2% BSA, 0.5% EDTA, 0.1% sodium azide to 50mM TRIS-HCL buffer at pH 7.2, and stir evenly to obtain R1 reagent.
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com