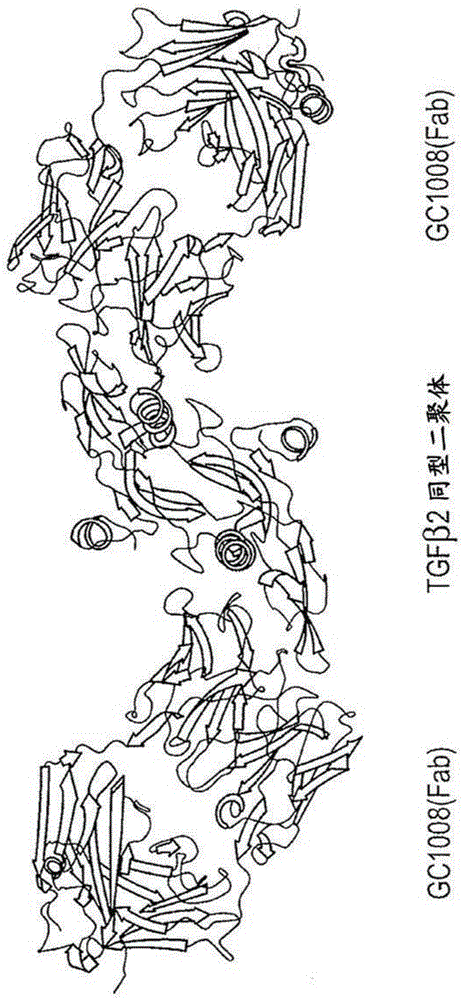
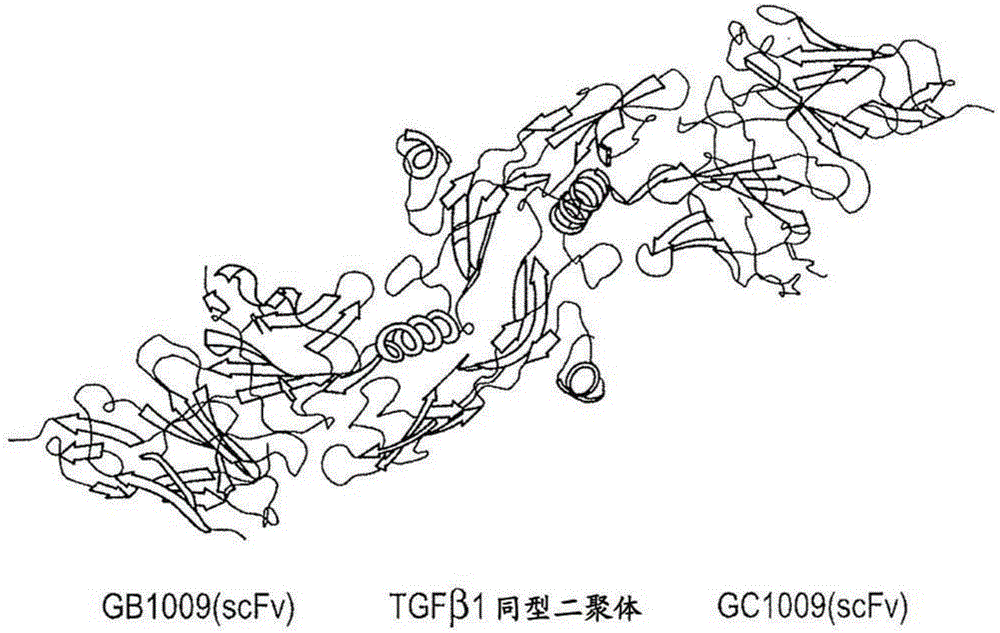
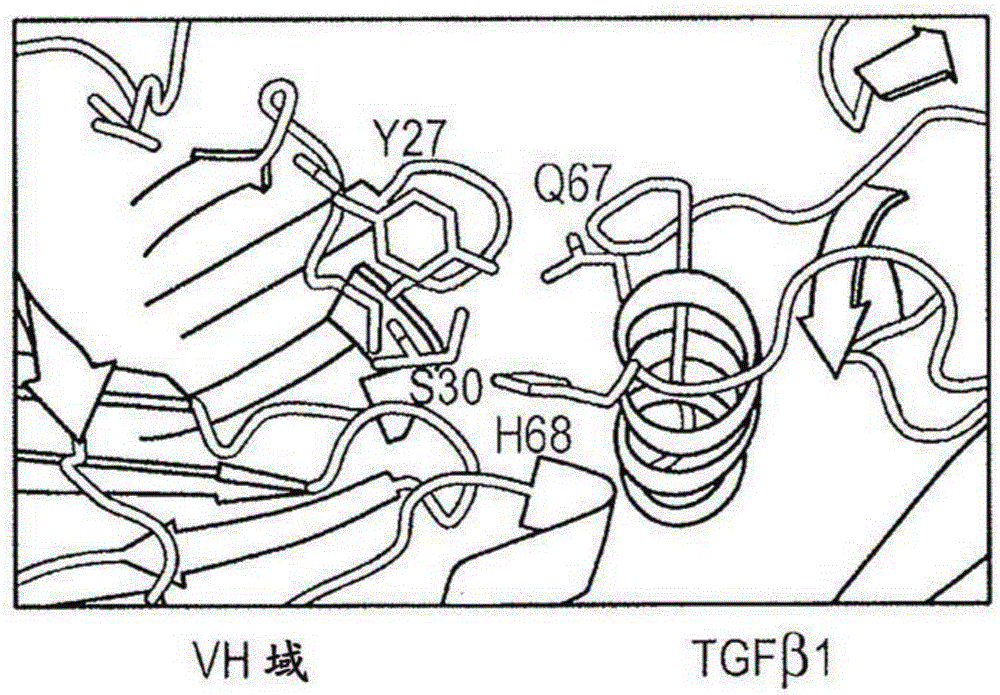
Engineered anti-tgf-beta antibodies and antigen-binding fragments
A technology that combines fragments and antigens, applied in the direction of antibodies, antibody medical components, drug combinations, etc., can solve the problem of reducing the survival rate of tumor cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0114] Anti-TGF beta single chain Fv (scFv) can be prepared according to the following non-limiting example disclosed in Example 1 of US Patent No. 7,723,486. Mutagenesis and / or combinatorial techniques can be used to increase the neutralizing potency for TGFβ1, TGFβ2 and / or TGFβ3. scFv with increased potency against TGFβ1, TGFβ2 and / or TGFβ3 can be generated by selecting and screening phage antibody libraries as described in Example 1 of US Patent No. 7,723,486. The scFv generated in that Example was compared to 1D11.16 disclosed in US Patent No. 7,723,486 in a MLEC proliferation assay.
[0115] In Example 1 of US Patent No. 7,723,486, a specific germline was found to be highly representative in the population of high potency TGFβ neutralizing scFvs. These are DP-10 / 1-69 and DP-88 / 1-e for the heavy chain (both members of the VH1 germline family), and DPK22 / A27 for the light chain (V κ 3 family). These germlines appear to provide a structural framework particularly suited f...
Embodiment 2A
[0119] The neutralizing potency of anti-TGF[beta] antibodies or antigen-binding fragments thereof can be determined using the TGF[beta]-dependent MLCE proliferation assay disclosed in Example 4 of US Patent No. 7,723,486. The MLEC proliferation assay is based on the assay described by Danielpour et al., J. Cell. Physiol., 138:79-86 (1989). This assay works on the principle that TGFβ1, TGFβ2 or TGFβ3 added to mink lung epithelial cells inhibit serum-induced cell proliferation. Antibodies were tested for neutralization of TGFβ1, TGFβ2 or TGFβ3, which resulted in restoration of cell proliferation. by ingesting [ 3 H]-thymidine to measure proliferation. Antibody potency was defined as neutralizing a single concentration of TGFβ1, TGFβ2 or TGFβ3 to a level of 50% (IC 50 ) antibody concentration (in nM).
[0120] MLEC Proliferation Assay Protocol: The MLCE cell line (Cat. #CCL-64) was obtained from the American Type Culture Collection. Cells were grown in minimal essential medi...
Embodiment 2B
[0129] Additionally, use The 3000 (GE Healthcare) instrument measures the TGFβ isoform binding affinity of the GC1008 antibody. TGFβ1 and TGFβ2 made in-house were diluted to ~1 μg / mL in 10 mM acetic acid, pH 4.5, while TGFβ3 (R&D Systems) was diluted to ~2 μg / mL in 10 mM acetic acid, pH 4.0. Flow cells 2, 3 and 4 of the CM5 sensor chip were covalently immobilized with 50 to 100 RU of TGFβ1, TGFβ2 and TGFβ3, respectively, using standard amine coupling kits from GE Healthcare. Flow cell 1 was used as a control surface. For kinetic binding assays, GC1008 was serially diluted 1:3 from 33.3 nM to 1.2 nM in HBS-EP buffer and injected into all four flow cells in triplicate for 5 min, then Dissociate in buffer at a flow rate of 30 μL / min for 5 min. The surface was regenerated with two 30 sec injections of 40 mM HCI at 75 μL / min. Sensorgrams were fitted using a 1 : 1 binding model after subtracting buffer versus control flow cell refractive index variations with the BIA Evaluation...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com