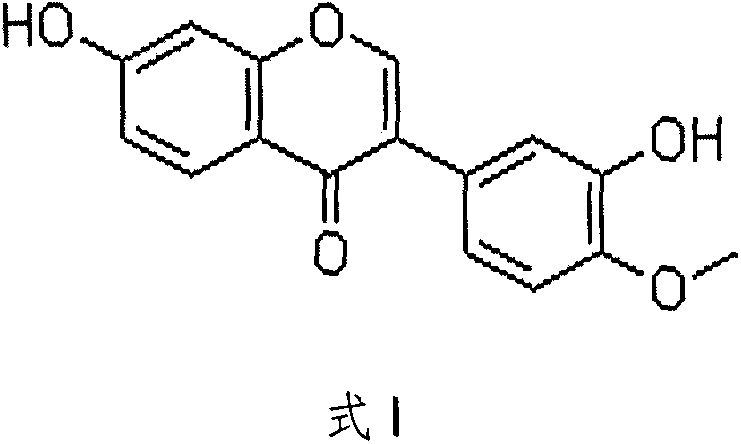
Application of calycosin in pharmacy
A technology for the use of mullein isoflavone, which is applied in the field of medical use of mullein, can solve the problems of changing the pathological process of allergic diseases and cannot reduce recurrence and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
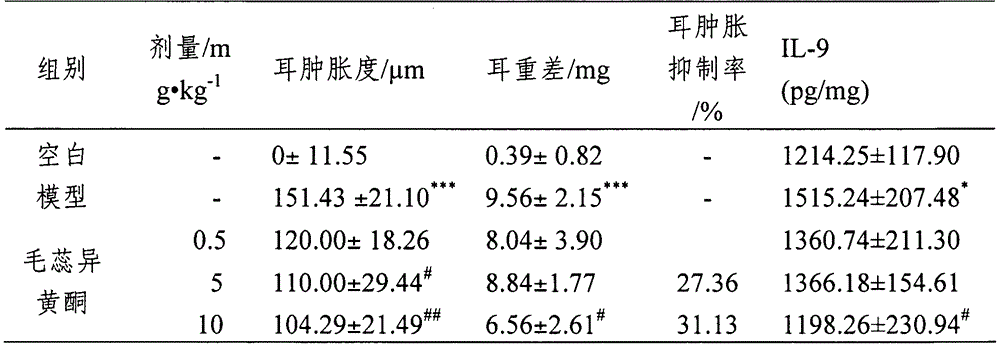
[0038] Methods: BALB / c mice were fed for 3 days after adaptive feeding, pre-administered for 2 days, the mice were shaved on the 0th day, and 1.5% FITC was applied to the hairless part of the abdomen on the 1st and 2nd days ( 80 μl of fluorescein isothiocyanate) solution was used for sensitization, and on the 6th day, 20 μl of 0.6% FITC solution was applied to the ears of mice (challenge), and the same volume of solvent was applied to the control group. After 24 hours of attack, measure the thickness of both ears of the mouse with a thickness gauge, and calculate the degree of ear swelling (right ear thickness-left ear thickness) and ear swelling inhibition rate=model group ear swelling-administration group ear swelling) / Model group ear swelling × 100%. After measuring the ear thickness, the left ear and the right ear were punched into ear pieces of the same size and weighed to calculate the difference in ear weight (weight of right ear - weight of left ear). After measuring...
Embodiment 2
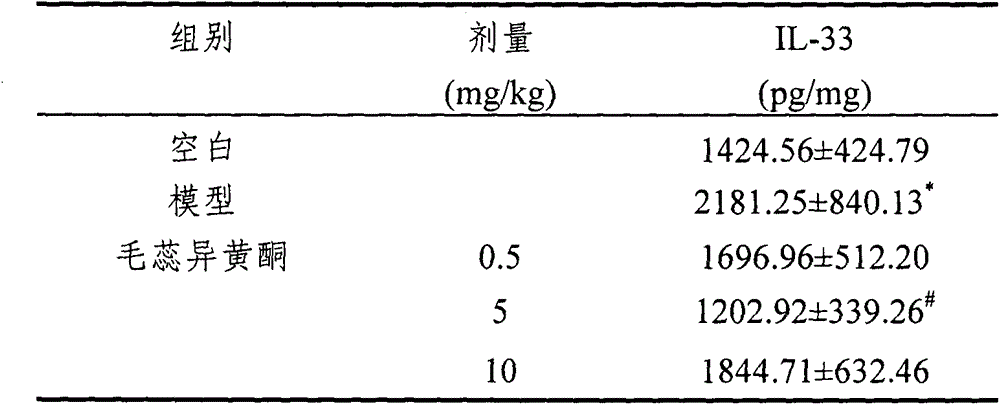
[0046] Compared with the blank group, the level of IL-33 in the ear tissue (the ear tissue was obtained from the modeling method described in Implementation 1) in the model group was significantly increased, and the effect of calycosin (5 mg / kg) on the IL-33 in the mouse ear tissue in the initial stage of sensitization produced a significant inhibitory effect. Other doses also had a certain downward trend in the production of IL-33.
[0047] Effect of calycosin on IL-33 in the initial stage of allergic disease sensitization
[0048] The effect of calycosin on IL-33 in the initial stage of allergic disease sensitization (mean±SD, n=8)
[0049]
[0050] *p<0.05, compared with the blank group; #p<0.05, compared with the model group;
[0051] Experiments have shown that calycosin can effectively treat allergic diseases by inhibiting the expression of IL-33 in vivo.
Embodiment 3
[0053] method:
[0054] a. Primary antibody (CaptureAntibody) coating: Dilute the CoatingBuffer (5×) with deionized water first, dilute the primary antibody with the diluted CoatingBuffer 250 times, 100 μl per well, then seal the plate with a sealing film, and incubate overnight at 4°C.
[0055] b. Pour off the CoatingBuffer on the board and pat dry with paper. Add ≥250 μl of washbuffer to each well, time for 1 min, and wash the plate five times.
[0056] c. The working solution (Assay Diluent) was diluted 5 times with deionized water, and 200 μl was added to each well. After the addition, affix a sealing film and incubate at room temperature for 1 hour.
[0057] d. Repeat operation b and wash the plate 5 times.
[0058] e. Sample addition: Dilute the standard product 1000 times with AssayDiluent to top, that is, 2000pg / ml, and then dilute the remaining 7 concentrations by 1 / 2. Add 100 μl of diluted standard and sample to each well, cover the plate with sealing film, and in...
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com