Application of triterpene glucoside or pharmaceutically acceptable salts thereof in preparation of tumor radiosensitizer
A technology of mogroside saponin and radiosensitizer, applied in the application field of mogroside saponin, to achieve the effect of improving radiosensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
[0064] This experiment verifies that the CASnumber is 88901-38-6 and the molecular formula is C 42 h 72 o 14 The radiosensitizing effect of mogroside Ⅱ on liver cancer cells.
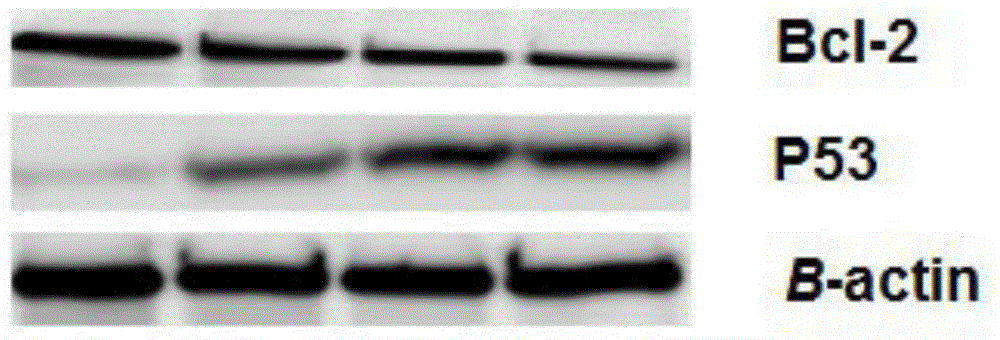
[0065] 1. WesternBlot method (ie WB method) was used to detect the effect of mogroside Ⅱ on P53 and Bcl-2 in liver cancer cells Hep-G2
[0066] Hep-G2 cells were treated with different concentrations of drugs (0 μmol·L -1 , 10 μmol L -1 , 30 μmol L -1 , 60 μmol L -1 ) was cultured for 24 hours, the cell culture was terminated, and the culture medium was sucked off, washed with PBS (concentration: 0.01 molL-1, pH 7.4), added 50 μL / well of lysate containing PMSF, and placed in an ice bath environment for lysis for 30 min. After 14000rmin -1 Centrifuge for 10 min at a high speed to obtain total protein. The protein concentration was measured by BCA colorimetry, 50 μg of total protein was taken, separated by 12% SDS polyacrylamide gel electrophoresis, electrotransferred to PVDF membrane, blocked wit...
Embodiment 2
[0083] This experiment verifies that the CASnumber is 88901-36-4 and the molecular formula is C 60 h 102 o 29 The radiosensitizing effect of mogroside V on lung cancer cells.
[0084] 1. Effect of mogroside V on P53 and Bcl-2 in lung cancer cells
[0085] The effect of mogroside Ⅴ on P53 and Bcl-2 was detected by protein immunoassay WesternBlot.
[0086] Hep-G2 cells were treated with different concentrations of drugs (0 μmol·L -1 , 10 μmol L -1 , 30 μmol L -1 , 60 μmol L -1 ) was cultured for 24 hours, the cell culture was terminated, and the culture medium was sucked off, washed with PBS (concentration: 0.01 molL-1, pH 7.4), added 50 μL / well of lysate containing PMSF, and placed in an ice bath environment for lysis for 30 min. After 14000rmin -1 Centrifuge for 10 min at a high speed to obtain total protein. BCA colorimetric method was used to measure protein concentration, 50 μg total protein was taken, separated by 12% SDS polyacrylamide gel electrophoresis, electr...
Embodiment 3
[0103] This experimental example verifies the CASnumber89590-98-7, the molecular formula is C 66 h 112 o 34 Radiation-sensitizing effect of mogroside Ⅵ on cervical cancer Hela cells.
[0104] clone forming experiment
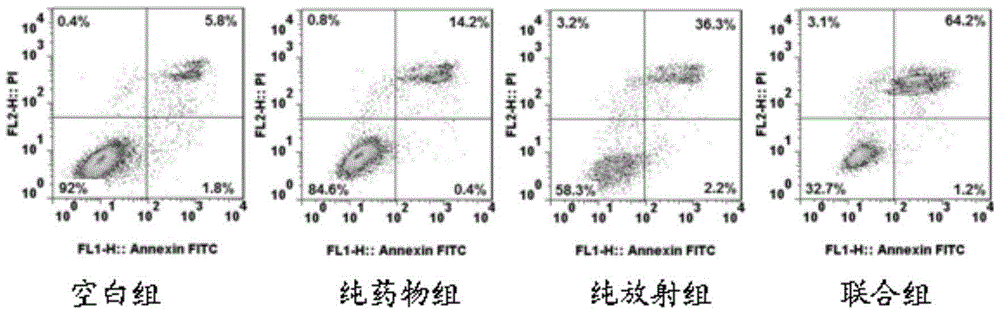
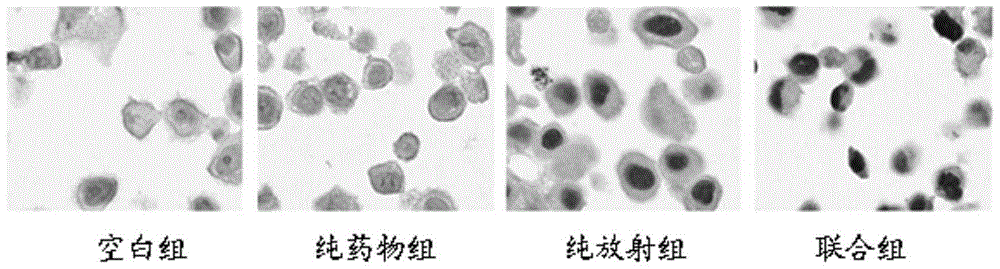
[0105] Cellular radiosensitivity was measured by colony formation assay. Dilute Hela cells to 1 x 10 4 / mL, added to 96-well plate, 100 μL per well. Divided into pure irradiation group, combined group (drug + irradiation group). Before irradiation, mogroside Ⅵ (concentration of 10 μmol / L in the combination group) was added to the treatment in the drug+irradiation group for 24 hours. Then, at room temperature, use 6MV-X-ray single irradiation, the doses are 0Gy, 2Gy, 4Gy, 6Gy, 8Gy respectively, irradiation conditions: 6MV-X-ray, room temperature irradiation, irradiation field area 15cm×15cm, plus 1.5cm, etc. Effective tissue filler. After irradiation, replace the culture medium and continue to incubate for 2 weeks, discard the supernatant of each well, fi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com