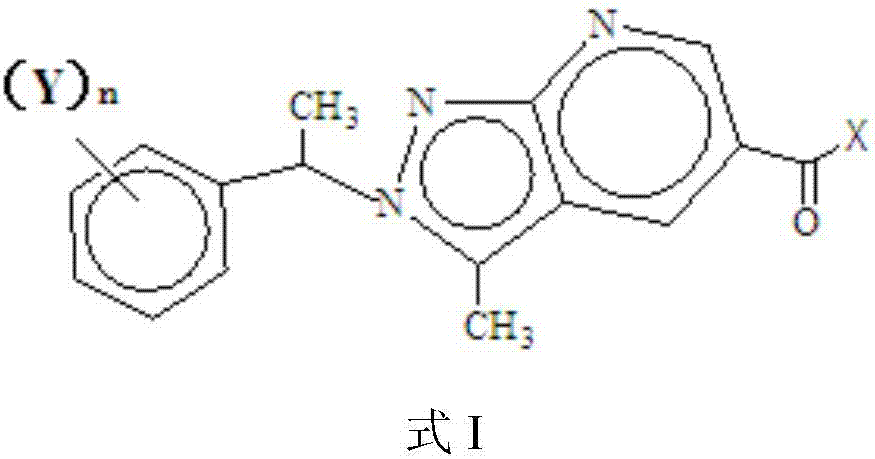
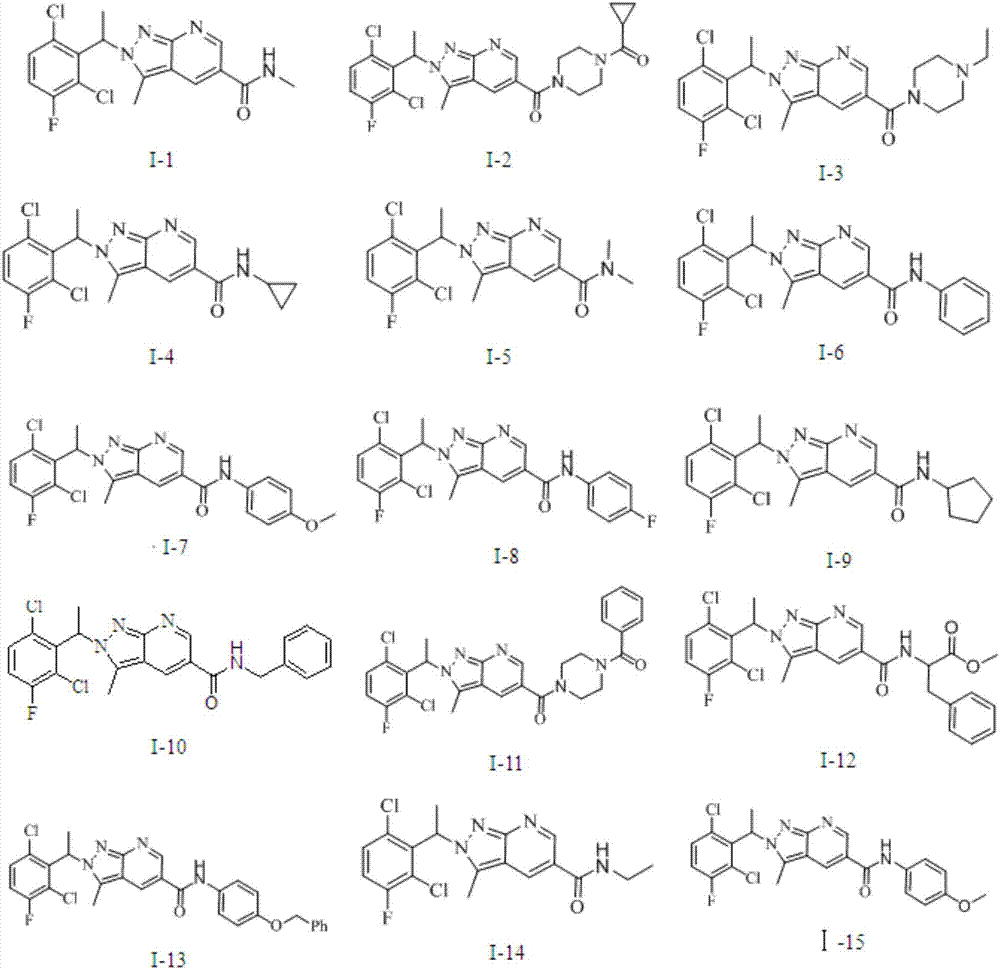
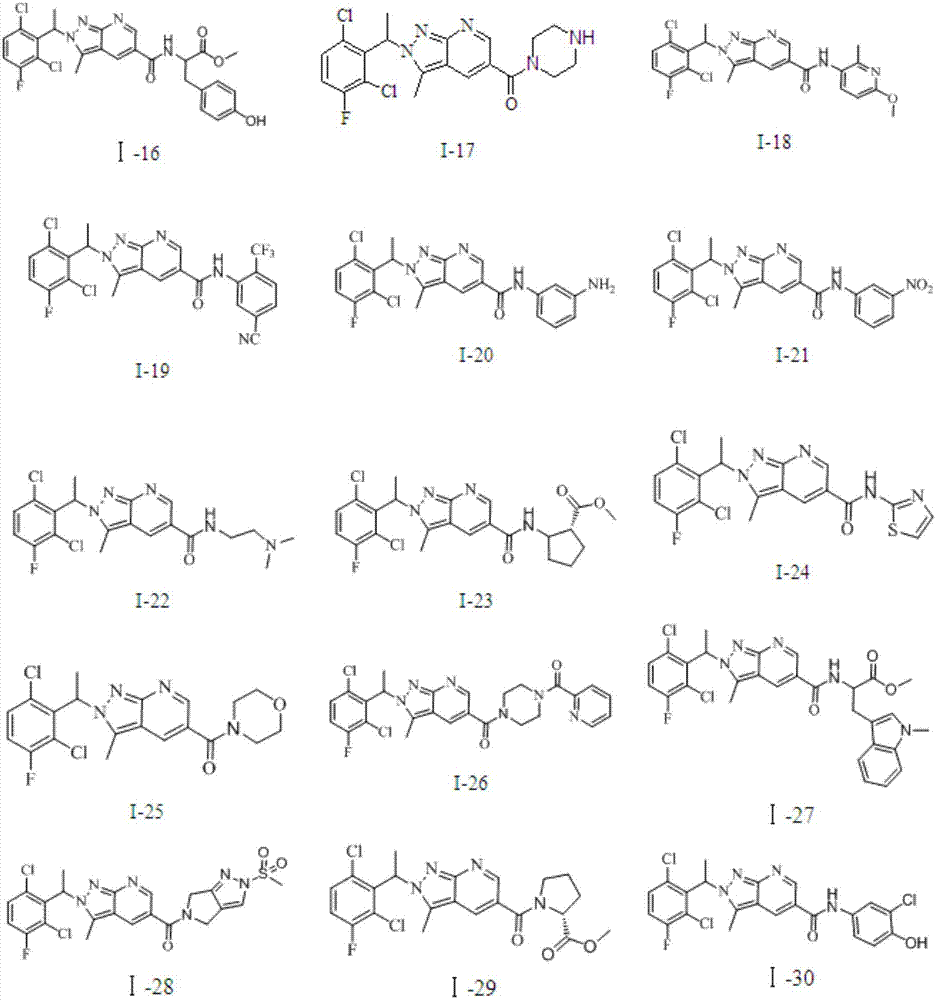
Pyrazolopyridine antitumor compound and its preparation method and application
A compound and selected technology, applied in the field of anti-tumor drugs, can solve the problems of unsatisfactory cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0077] The preparation of embodiment 1 compound I-1
[0078] first step:
[0079]
[0080] Add 40g of raw material i into a 2L single-necked bottle, add 800mL of dichloromethane to dissolve it, then add 38.6g of triethylamine, add 32.6g of methanesulfonyl chloride dropwise at low temperature, and return to room temperature for reaction after dropping. After the reaction was completed, it was washed twice with 1 mol / L hydrochloric acid, once with saturated sodium chloride, dried over anhydrous sodium sulfate, and spin-dried to obtain 55 g of a brown-red oily liquid.
[0081] Step two:
[0082]
[0083] Add 64.6g of intermediate II and 180ml of DMF into a 2L single-necked bottle, add 41.6g of III after dissolving, then add 74g of cesium carbonate, and heat the oil bath until the external temperature is 105oC. After the reaction was completed, a large amount of water was added, extracted with ethyl acetate, spin-dried and separated by column chromatography to obtain 50 g ...
Embodiment 2
[0092] The preparation of embodiment 2 compound Ⅰ-2
[0093]
[0094] Add 1.0g of intermediate VI and 0.62g of cyclopropyl-piperazin-1-yl-methyl ketone into a 100mL one-necked bottle, then add 10mL of DMF and stir to dissolve, then add 2.05g of HBTU, 0.82g of triethylamine, and react at room temperature for 5h . After the reaction, the reaction solution was poured into water, extracted with ethyl acetate, dried with anhydrous sodium sulfate and spin-dried, and separated by column chromatography to obtain 1.1 g of solid.
[0095] 1 HNMR(DMSO-d6)δ:8.34(1H,s),8.05(1H,s),7.18~7.15(1H,m),6.97~6.92(1H,m),6.51~6.46(1H,q),3.67 ~3.60(8H, m), 2.54(3H, s), 2.11(3H, d, J=7.2Hz), 1.66~1.64(1H,m), 0.94~0.86(2H,m), 0.73~0.71(2H ,m).
[0096] m / z=505(M+H+)
[0097] The preparation of Examples 3-32 refers to the process route above in the specification, and the preparation process of the compound is similar to that of Example 1 and Example 2. Table 1 below shows the test data of the e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com