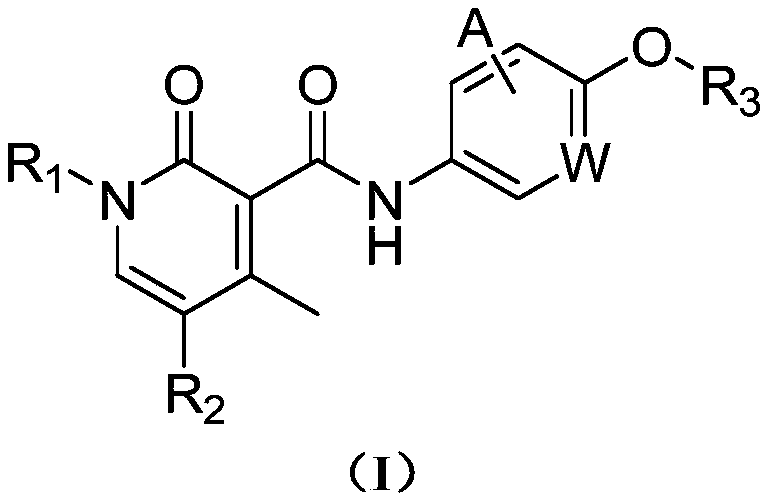
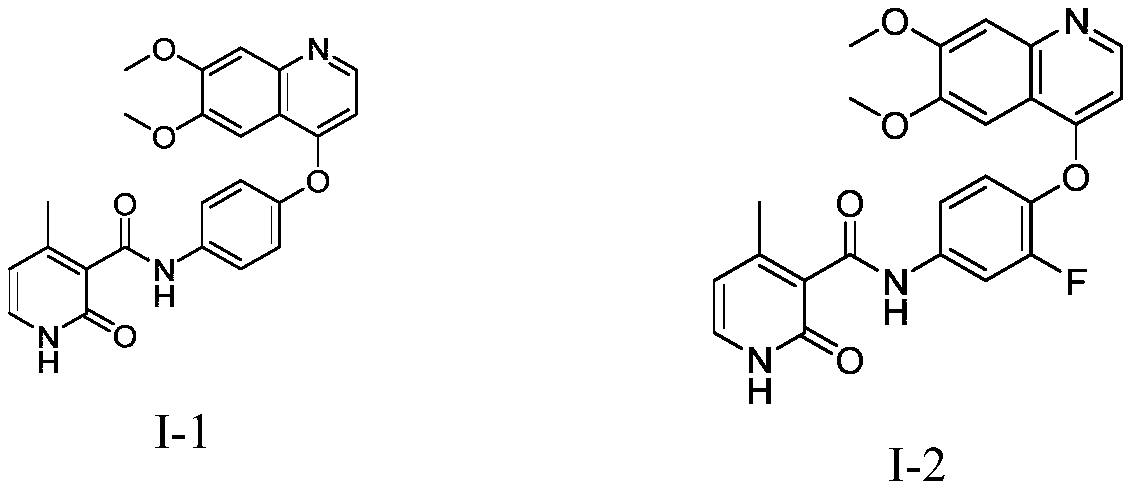
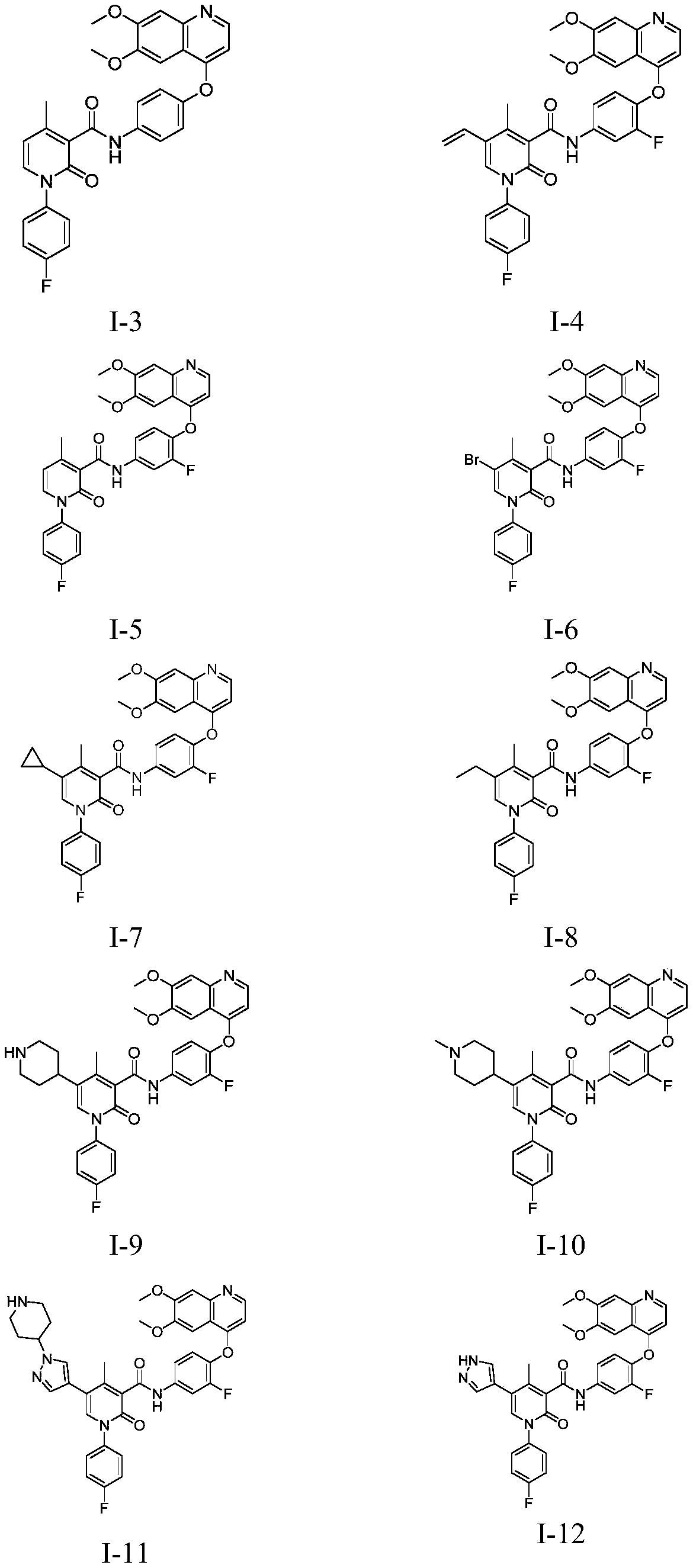
Antitumor compound used as AXL inhibitor and application of antitumor compound
A compound, drug technology, applied in the field of medicinal chemistry
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0076] Example 1 Compound 5 Synthesis
[0077]
[0078] Synthesis of Compound 1: Take a 1.0L three-necked flask, add 100.0g of acetylacetaldehyde dimethyl acetal, 11.0g of piperidinium acetate and 400ml of toluene, then slowly add 50.0g of malononitrile, react at 35°C for 16.0h, After the reaction finished, add 200ml of water to wash, separate the layers, keep the organic phase, dry with anhydrous sodium sulfate, and concentrate under reduced pressure to dryness to obtain 130.0g compound 1, which is propylene malononitrile and propenylene malononitrile ( 10:1) mixture.
[0079] Synthesis of Compound 2: Take a 250ml three-neck flask, add 70ml of concentrated sulfuric acid, stir at 0°C, slowly add compound 1 dropwise, after the addition is complete, raise the temperature to 50°C for 5.0 hours, after the reaction, drop to 0°C, add dropwise to In 200ml of 0°C water, a solid precipitated out, and it was filtered with suction. The filter cake was rinsed with 50ml of water, drain...
Embodiment 2
[0083] The preparation of embodiment 2 compound I-1
[0084]
[0085] I-1-1 Synthesis: Take a 100ml three-necked flask, add 2.23g 4-chloro-6,7-dimethoxyquinoline, 1.63g p-aminophenol, 1.44g sodium tert-butoxide, 10ml DMAC, and react at 105°C After 12.0 hours, the reaction of the raw materials was basically complete, poured into 100ml of water, and filtered with suction to obtain a brown-black solid, and 2.05g of compound I-1-1 was obtained by column chromatography.
[0086] Synthesis of I-1: Take a 100ml three-neck flask, add 300mg I-1-1, 150mg compound 3, 570mg HBTU, 390mg DIEA and 10ml DMF in sequence, react at 25°C for 3.0h, the raw materials are basically reacted completely, add the reaction solution into 100ml water, and filter with suction , filter cake column chromatography to obtain 15 mg of compound I-1, MS m / z=432.2 (M+1), 1 H NMR (400MHz, d6-DMSO) δ: 10.83(s, 1H), 8.49-8.48(m, 1H), 8.21-8.18(m, 1H), 7.99-7.98(m, 1H), 7.55-7.53(m , 1H), 7.45-7.41 (m, 4H), 7.22-7...
Embodiment 3
[0087] The preparation of embodiment 3 compound 1-2
[0088]
[0089] I-2-1 Synthesis: Take a 100ml three-necked flask, add 2.23g 4-chloro-6,7-dimethoxyquinoline, 1.91g 4-amino-2-fluorophenol, 1.44g sodium tert-butoxide, 10ml DMAC , reacted at 105°C for 12h, the raw materials were basically reacted completely, poured into 100ml of water, and filtered with suction to obtain a brown-black solid, and column chromatography gave 1.50g of compound I-2-1.
[0090] I-2 Synthesis: Take a 100ml three-necked flask, add 315mg of compound I-2-1, 150mg of compound 3, 570mg of HBTU, 390mg of DIEA, and 10ml of DMF in sequence, and react at 25°C for 3.0h. The raw materials are basically reacted completely, and the reaction solution is added to 100ml of water , a large amount of solids precipitated, suction filtration, filter cake column chromatography to obtain 20 mg of compound I-2, MS m / z=450.5 (M+1), 1 HNMR (400MHz, d6-DMSO) δ: 10.82(s, 1H), 8.52-8.50(m, 1H), 8.03-8.02(m, 1H), 7.94-7.92...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com