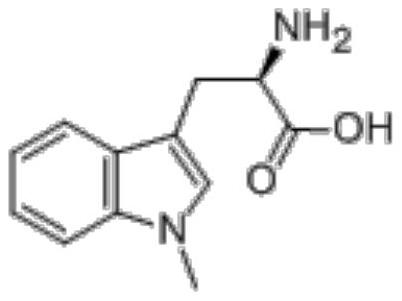
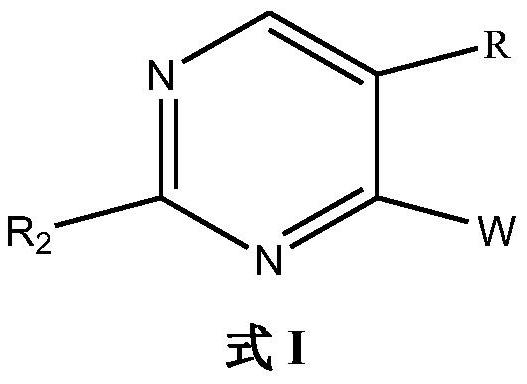
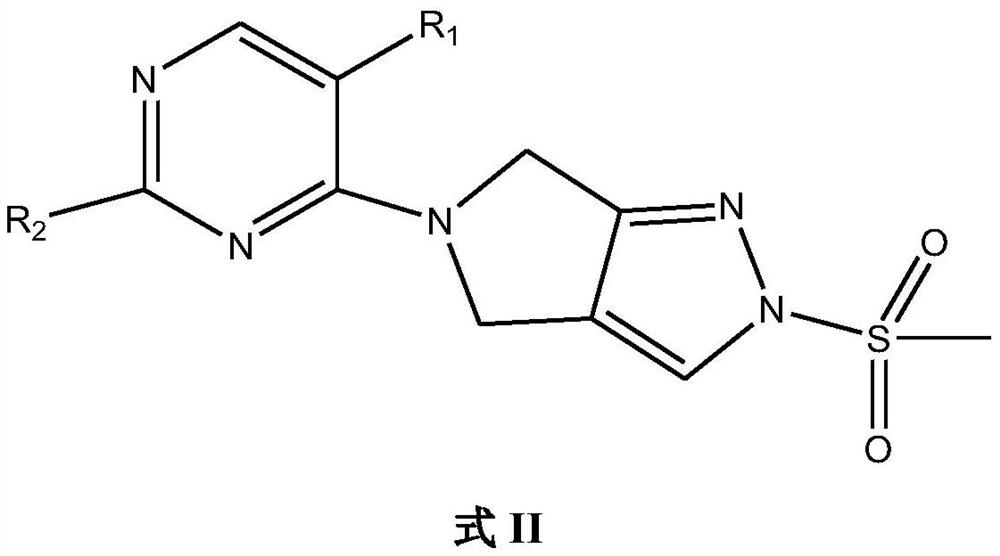
Ido inhibitor and preparation method and application thereof
A technology of phenyl and compound, applied in the field of IDO inhibitor and its preparation, can solve the problem of unsatisfactory inhibition effect of IDO inhibitor
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0108] Embodiment 1: the preparation of compound I-1
[0109]
[0110] Synthesis of compound 7:
[0111]Take a 100ml three-necked flask, add 1.23g of 5-bromo-2,4-dichloropyrimidine, add 5ml of ethanol to dissolve it, put it in a refrigerator under nitrogen protection, add 0.54g of triethylamine at 0°C, and take 1.5g of 2 , 4,5,6-tetrahydro-2-(methylsulfonyl)pyrrolo[3,4-C]pyrazole, after adding 20ml of ethanol to dissolve it, add it dropwise to the above reaction solution at 0°C, the reaction solution Solids were precipitated in the solution, and the low-temperature reaction was continued for 2 h after the addition was completed. TLC detected that the reaction of the raw materials was complete, and the reaction was stopped, and 1.74 g of off-white solids were obtained by suction filtration, with a yield of 85%.
[0112] Synthesis of compound I-1:
[0113] Add 0.20g compound 1, 0.13g 4-fluoro, 3-trifluoromethylbenzeneboronic acid, 0.06gPd(dppf)Cl to a 100ml single-necked bo...
Embodiment 2
[0115] The synthesis of embodiment 2 compound I-48
[0116]
[0117] Preparation of intermediate 8 compound:
[0118] Weigh 0.60g of compound 1 in a 100mL reaction bottle, then weigh 0.26g of 3-thiophene boronic acid, 0.14g of Pd(dppf)Cl 2 And 0.66g of potassium carbonate, finally added 10mL of DMA and 1mL of water, replaced with nitrogen three times and placed in an oil bath, raised to 80 ° C for 1 h. TLC detected that the reaction was complete, quenched by adding 100mL of water, extracted with 50ml*2EA, combined the organic layers and dried with anhydrous sodium sulfate, spin-dried under reduced pressure, column chromatography (petroleum ether: ethyl acetate), and obtained 300mg of the final product as intermediate Body 2, with a purity of 99%.
[0119] Synthesis of Compound I-48:
[0120] Weigh 0.29g of intermediate 8 in a 100mL reaction flask, then weigh 0.28g of 4-fluoro-3-trifluoromethylbenzeneboronic acid, 0.10g of Pd(dppf)Cl 2 And 0.36g potassium carbonate, fina...
Embodiment 3
[0121] The synthesis of embodiment 3 compound I-52
[0122]
[0123] Synthesis of Compound 9: Take a 100ml single-necked bottle, add 5.0g Fmoc proline, 0.5g methylamine, 3.2g EDCI and 3.0g triethylamine, 50ml dichloromethane to dissolve and react at room temperature for 3 hours, TLC detects that the reaction of the raw materials is complete, stop To react, add 50ml of DCM to dilute, wash with 25ml*2 dilute HCl, 50ml*2 saturated sodium chloride, dry over anhydrous sodium sulfate, spin dry under reduced pressure to obtain a crude product, and column chromatography to obtain 4.5g white solid.
[0124] Synthesis of Compound 10: Take a 100ml single-necked bottle, add 4.5g of Compound 3, 2ml of pyridine and 10ml of DMF in sequence, and react at room temperature for 2 hours, TLC detects that the raw materials are completely reacted, stop the reaction, add 50ml of DCM to dilute, wash with 50ml*2 saturated sodium chloride, Dry over anhydrous sodium sulfate, spin dry under reduced pr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com