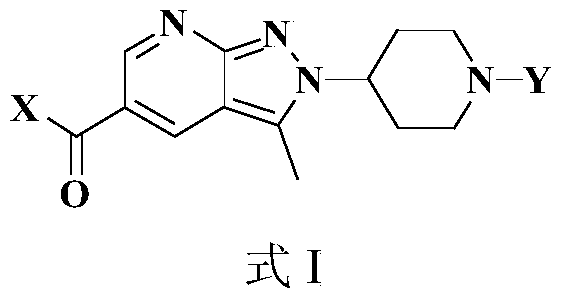
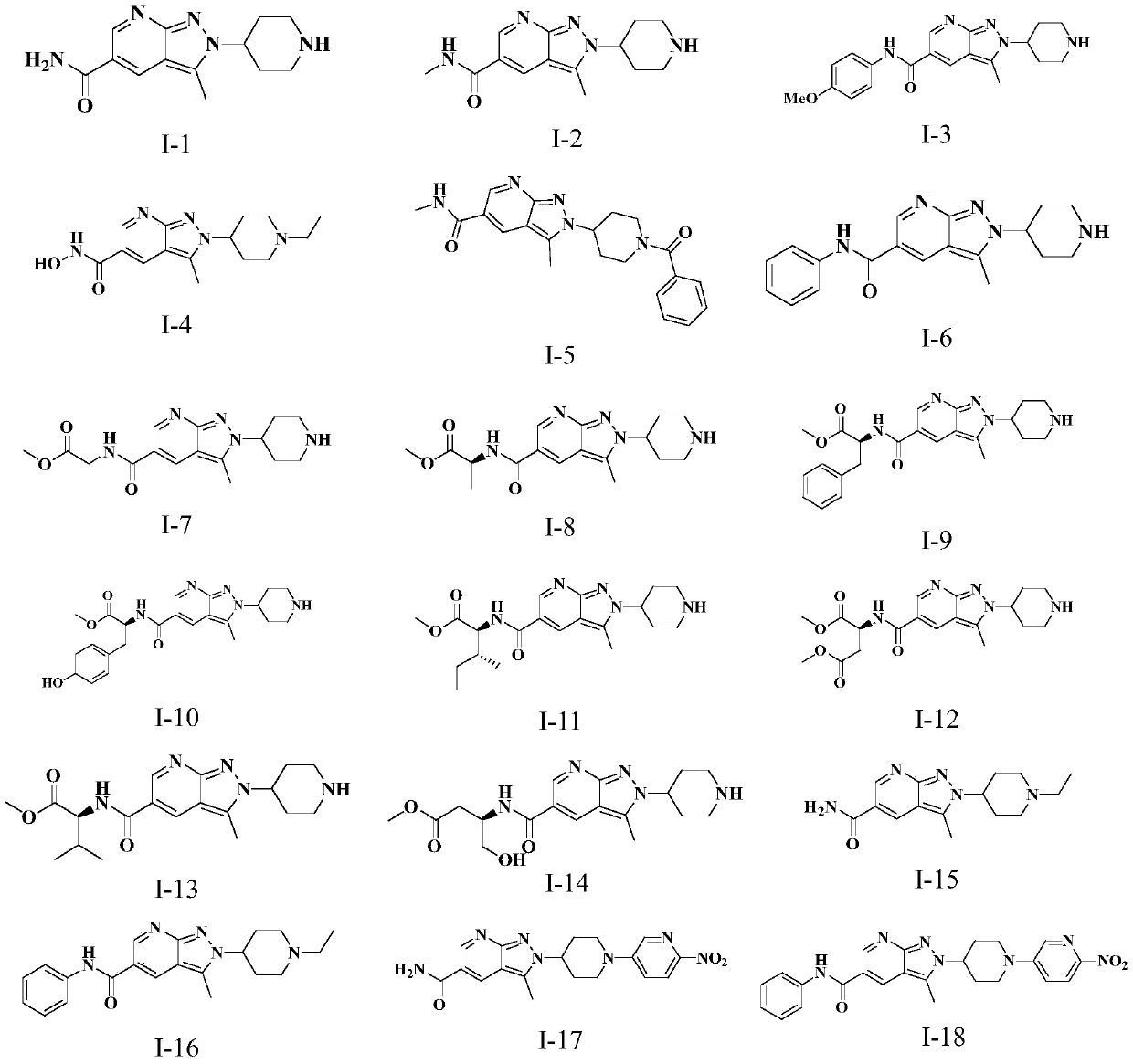
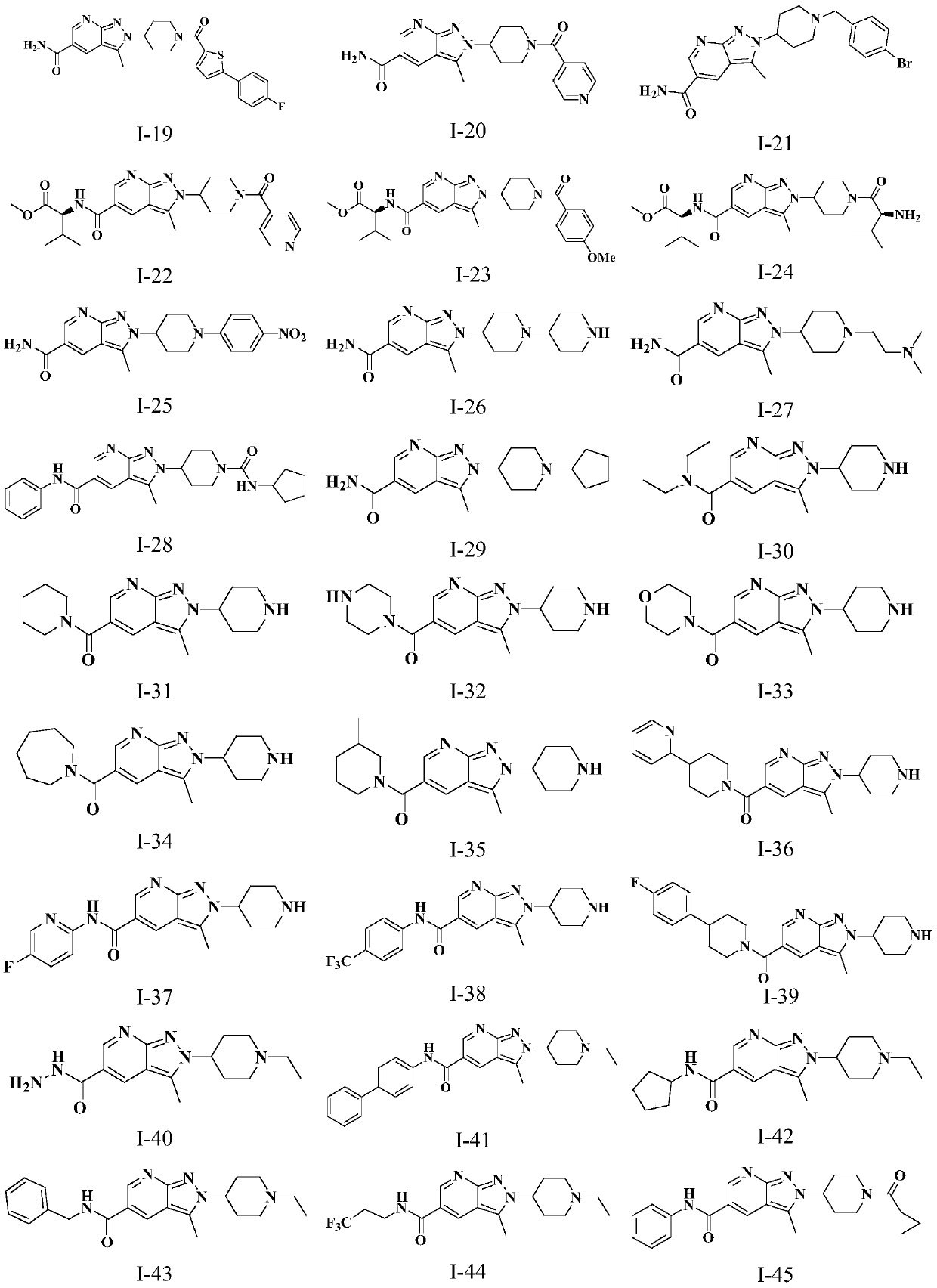
Novel anti-tumor compound
A technology of compounds and hydrates, applied in the direction of antineoplastic drugs, active ingredients of heterocyclic compounds, drug combinations, etc., can solve problems such as unsatisfactory cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0103] The preparation of embodiment 1 compound I-1
[0104]
[0105] Throw T-160g, add 250ml methanol to dissolve, then slowly add NaBH 4 , keeping the temperature below 10° C., TLC monitored the complete reaction of the raw materials to obtain about 60.5 g, with a yield of 99.8%.
[0106]
[0107] Throw T-260g, add dichloromethane to dissolve, then add triethylamine, place below 0°C, add MeSO2Cl dropwise, TLC monitors the reaction of raw materials is complete. About 80 g was obtained after post-processing, and the yield was 96%.
[0108]
[0109] Throw in 13.6g of T-3, dissolve it with 100ml of DMF, then add 1.0eq of compound T-4 and 1.5eq of cesium carbonate, heat up to 100°C for reaction, and monitor the complete reaction of raw materials by HPLC. About 8 g of T-5 was obtained by column chromatography, with a yield of 42.37%.
[0110]
[0111] Throw 4g of T-5, dissolve it with 40ml of ethanol, add 40ml of water and 2eq of sodium hydroxide, stir at room tempe...
Embodiment 2
[0118] The preparation of embodiment 2 compound 1-2
[0119]
[0120] Throw 0.31g of T-6, add 20ml of DMF to dissolve, 1.5eq of methylamine hydrochloride, 3eq of triethylamine and 1.5eq of HBTU, stir and react at room temperature for 24h, TLC detects that the reaction is complete, pour the reaction into 200ml of water, acetic acid Extracted with ethyl ester, dried with anhydrous sodium sulfate, filtered, spin-dried, passed through the column to obtain 0.21 g of white solid, yield 71%
[0121]
[0122] Throw 0.21g of T-7, dissolve it with 5ml of methanol, add 2ml of concentrated hydrochloric acid, stir at room temperature for 3 hours, TLC detects that the raw materials have reacted completely, spin dry, add water to dissolve, adjust the pH value to 8-9, extract with ethyl acetate, and dry with anhydrous sodium sulfate , filtered and spin-dried to obtain 0.12 g of a white solid, with a yield of 80%.
[0123] 1 HNMR(DMSO-d6)δ:9.04(d,J=2Hz,1H),8.75(d,J=2Hz,1H),5.80~5.63(br...
Embodiment 3
[0125] The preparation of embodiment 3 compound 1-3
[0126]
[0127] Add compound T-58g, add 40ml of absolute ethanol, add 16ml of concentrated hydrochloric acid, stir at room temperature for 6 hours, TLC detects that the raw materials have reacted completely, spin dry, add water to dissolve, adjust the pH value to 8-9, extract with ethyl acetate, anhydrous sodium sulfate After drying, filtering and spin-drying, 5.4 g of white solid was obtained, with a yield of 85%.
[0128]
[0129] Throw C-12.9g, dissolve with 30ml of DMSO, then add 1.5eq of potassium carbonate, 0.1eq of potassium iodide, 1.2eq of bromoethane and 0.1eq of tetrabutylammonium bromide, stir the reaction at 60°C, after Workup yielded about 1.9 g, yield 60.1%.
[0130]
[0131]Throw 1.5g of C-2, dissolve it with 20ml of ethanol, add 20ml of water and 2eq of sodium hydroxide, stir at room temperature for 12h, TLC detects that the reaction is complete, add 200ml of water, adjust the pH value to 5-6, a l...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com