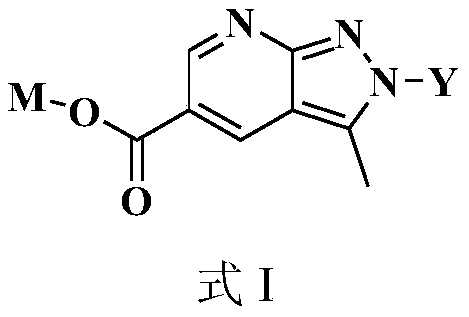
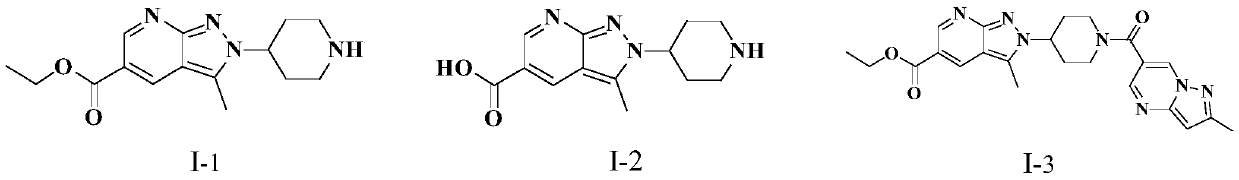
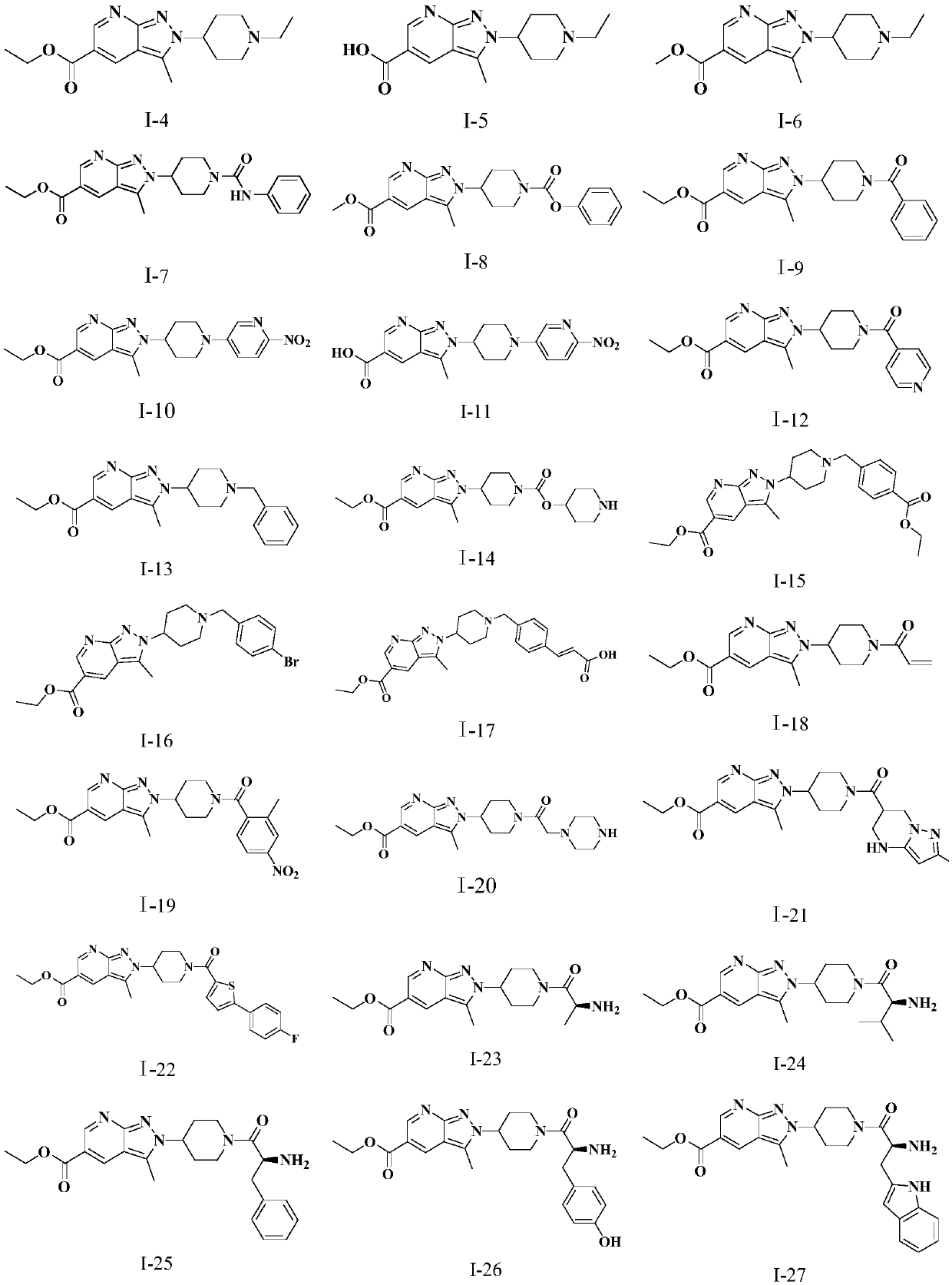
Novel pyrazolopyridine antineoplastic compound
A compound and selected technology, applied in the field of anti-tumor drugs, can solve the problems of unsatisfactory cancer patients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0087] The preparation of embodiment 1 compound I-1
[0088]
[0089] Put in the raw material T-160g, add 250ml of methanol to dissolve it, and then slowly add 0.35 molar equivalent of NaBH 4 , keep the temperature below 10°C to obtain about 60.5g, yield 99.8%.
[0090]
[0091] Put in 260g of raw material T-260g, add 300ml of dichloromethane to dissolve it, then add 1.5 molar equivalent of triethylamine (TEA), place below 0°C, and dropwise add 1.5 molar equivalent (eq) of methanesulfonyl chloride. About 80 g was obtained after post-processing, and the yield was 96%.
[0092]
[0093] Put in 13.6g of raw material T-3, add 100ml of DMF, then add 1.0eq of T-4 and 1.5eq of cesium carbonate, and heat up to 100°C for reaction. About 8 g was obtained by post-processing column chromatography, and the yield was 42.37%.
[0094]
[0095] Put in 13.6g of raw material T-3, add 100ml of absolute ethanol, add 25ml of concentrated hydrochloric acid dropwise at low temperature...
Embodiment 2
[0096] The preparation of embodiment 2 compound 1-2
[0097] 3-Methyl-2-piperidin-4-yl-2H-pyrazolo[3,4-b]pyridine-5-carboxylic acid
[0098]
[0099] Put in 1-10.5g of raw materials, add 10ml of absolute ethanol, add 1.5g of sodium hydroxide and dissolve it in water, and then add it to react at room temperature. After post-treatment, about 0.34 g was obtained, with a yield of 85%. 1 HNMR (DMSO-d 6 )δ9.45(br,1H),9.02(d,J=2Hz,1H),8.77(d,J=2Hz,1H),7.41(br,1H),5.19~5.11(m,1H),3.41~ 3.21(m,2H), 3.19~3.11(m,2H), 2.57(s,3H), 2.46~2.30(m,2H), 2.12~2.04(m,2H).m / z=261(M+H + ).
Embodiment 3
[0100] The preparation of embodiment 3 compound 1-3
[0101]
[0102] Add 10.29g of raw material I-7, 1.0eq of compound T-7, 1.5eq of amidation reaction catalyst HBTU, 1.5eq of TEA, and 10ml of DMF, and stir the reaction at room temperature for 24h. After post-treatment, about 0.21 g was obtained, with a yield of 46.67%. 1 HNMR (DMSO-d 6 )δ: 9.13(d, J=2Hz, 1H), 8.79(d, J=2Hz, 1H), 8.67(d, J=2Hz, 1H), 8.54(d, J=2Hz, 1H), 6.54(s ,1H),5.12(m,1H),4.42(m,2H),3.29~3.03(m,4H),2.60(s,3H),2.57(s,3H),2.46~2.30(m,2H), 2.12~2.04(m,2H),1.42(m,3H),.m / z=448(M+H + )
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com