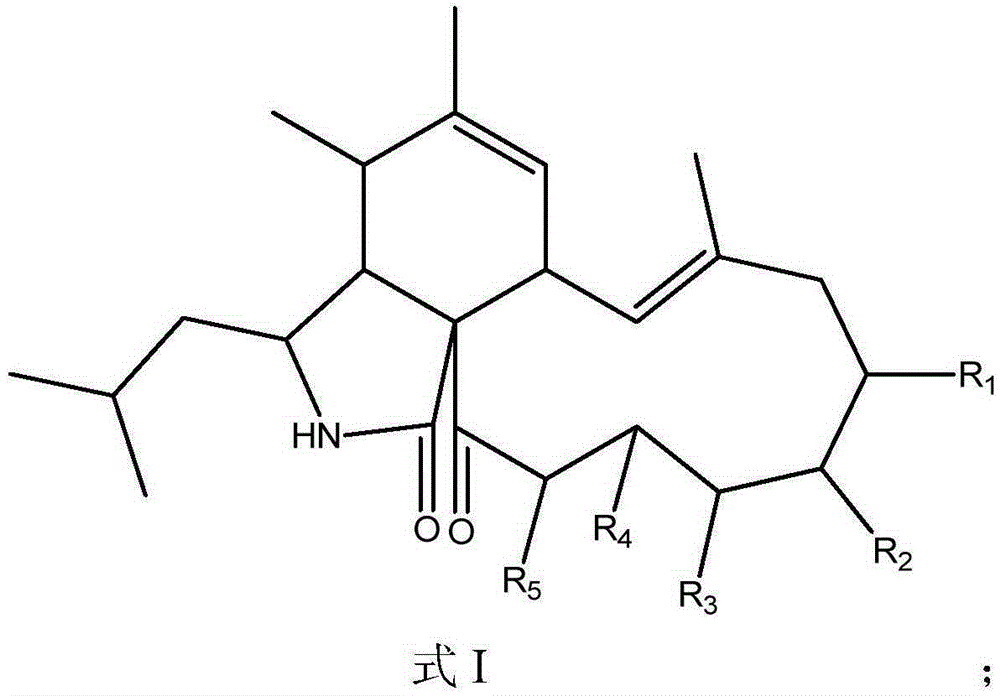
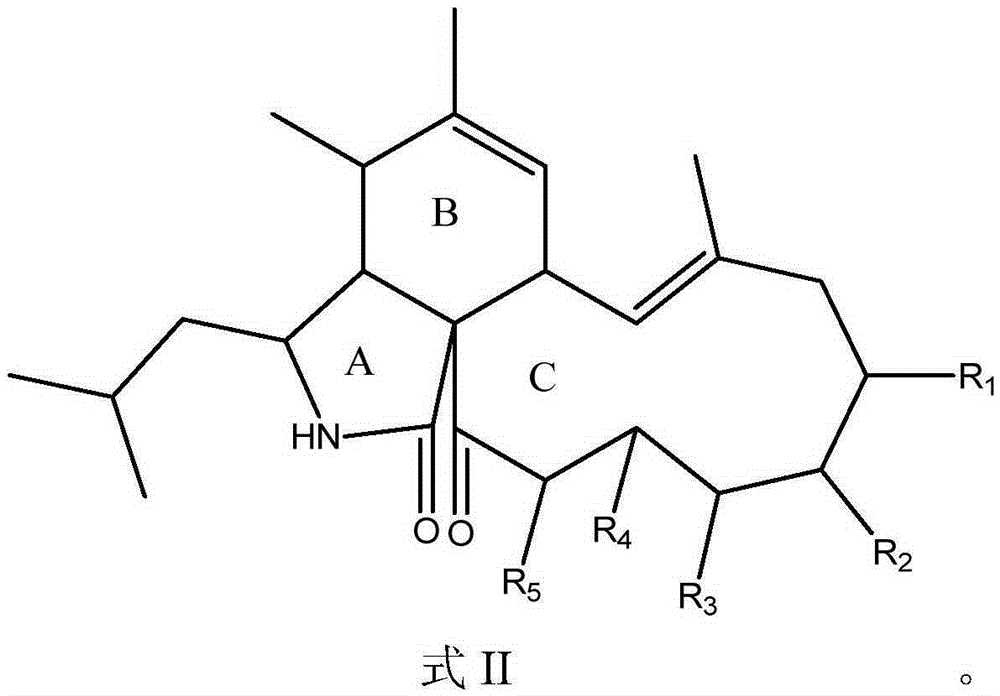
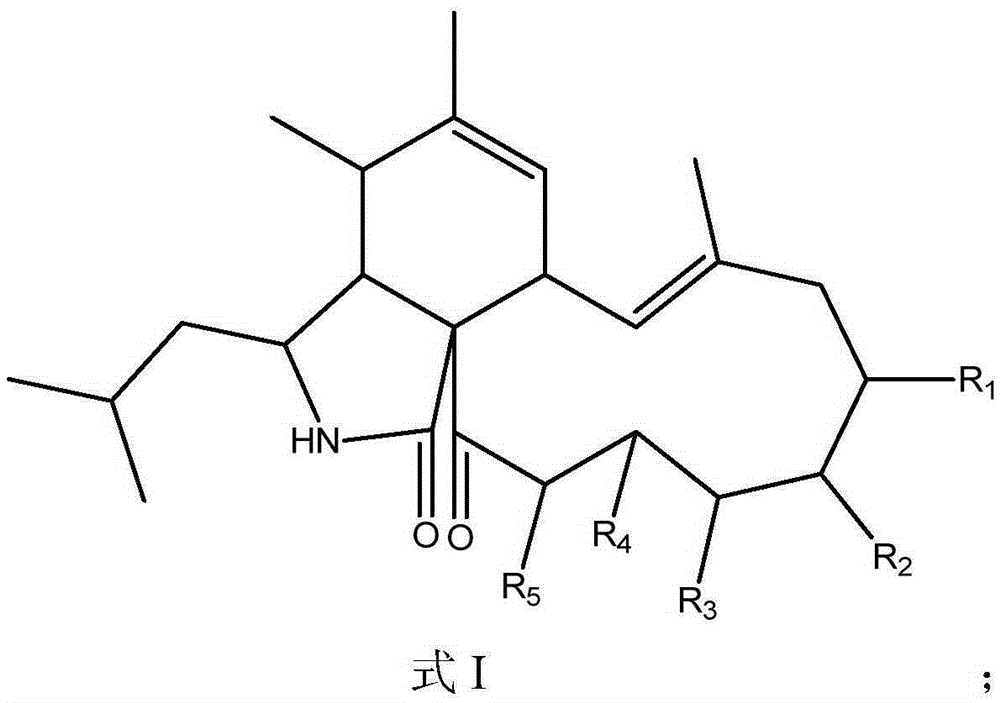
Application of Aspochalasin compounds to preparation of anti-HIV (human immunodeficiency virus)-latency drugs and acquired immune deficiency syndrome treating drugs
A compound and AIDS technology, applied in the field of preparation of anti-HIV latent drugs and AIDS drugs, can solve the problems of weak activation effect and obvious adverse reactions, and achieve the effect of accelerated clearance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] HIV Latency Induction Activation Experiment
[0021] The cells used for activity screening were divided into 2 × 10 per well 4Each was planted in a 96-well plate, and 100 microliters of 1640 medium (Gibco) containing 10% FBS (fetal bovine serum, Gibco) was added to each well. After 24 hours, a certain concentration of Aspochalasin compounds was added. After the cells were treated with drugs for 72 hours, the expression of GFP in the cells was observed under a fluorescence microscope, and the cells were collected for flow cytometry detection to analyze the proportion of fluorescent cells.
Embodiment 2
[0023] With a final concentration of 20 μg / ml AspochalasinE (R 1 , R 2 , R 3 , R 4 and R 5 Treat HIV latently infected cell models with OH, OH, OH, H and H) respectively, and analyze the activation of HIV latently infected cells by fluorescent microscope observation and flow cytometry detection of the reporter gene green fluorescent protein after 3 days of its action Efficiency, the results showed that the relative activation rate of HIV latently infected cells was 62% after AspochalasinE treatment.
Embodiment 3
[0025] With final concentration of 20 μg / ml aspochlasinC (R 1 , R 2 , R 3 Respectively H, OH, OH, R 4 and R 5 Forming double bonds) to treat the HIV latent infection cell model, after 3 days of its action, through the fluorescent microscope observation and flow cytometry detection of the reporter gene green fluorescent protein, the activation efficiency of HIV latent infection cells was analyzed, the results showed that HIV latent infection The relative activation rate of the cells after treatment was 80%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com