Cooperative roasting method for zinc concentrate and iron pyrite
A technology for pyrite and zinc concentrate, which is applied in the intersection of metallurgical engineering and environmental engineering, can solve the problems of no economic benefit, environmental harm, loss of pyrite slag, etc., and achieves simple operation, environmental friendliness, and short technological process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
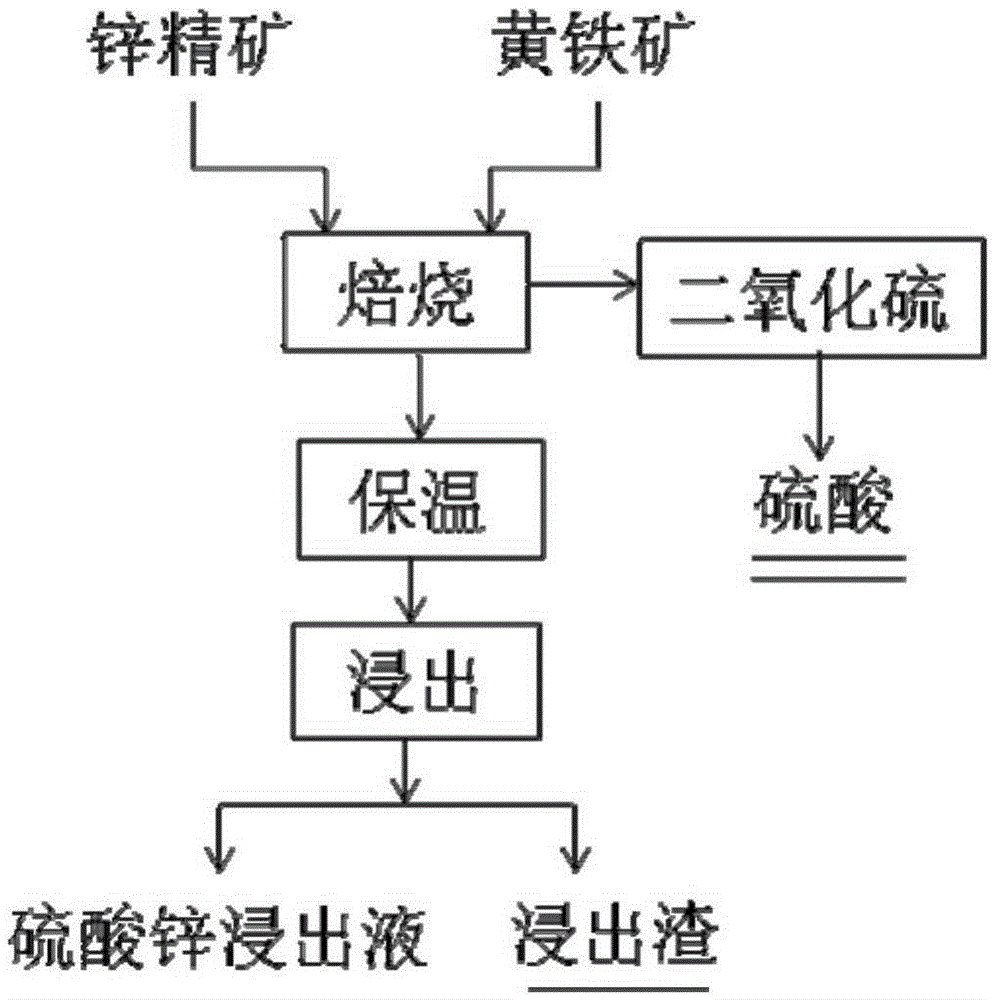
[0019] Pyrite and zinc concentrate are mixed evenly in a mass ratio of 1, and pure oxygen is introduced to carry out boiling roasting at 800 ° C. After boiling roasting, the temperature is kept at 650 ° C for 60 minutes to obtain zinc calcined sand. The leaching agent is sulfuric acid content 90g / L, 80℃, liquid-solid ratio is 10 / 1, leaching time is 30min, after leaching is completed, filter and separate to obtain zinc sulfate leaching solution, zinc leaching rate is 90.12%, copper content in zinc sulfate leaching solution is 0.8g / L, iron content It is 0.04g / L. Table 1 shows the contents of zinc sulfide and zinc ferrite in the zinc calcine obtained in the present invention and the traditional zinc smelting zinc calcine.
[0020] Table 1 Zinc sulfide and zinc ferrite content in zinc calcine
[0021] condition
[0022] There is pyrite
Embodiment 2
[0024] Pyrite and zinc concentrate are mixed evenly in a mass ratio of 2, and pure oxygen is introduced to carry out boiling roasting at 900 °C. After boiling roasting, the temperature is kept at 620 °C for 60 minutes, and the obtained calcined sand is leached at 80 °C. The sulfuric acid content of the leaching solution is 90g / L, the liquid-solid ratio is 10 / 1, and the leaching time is 30min. After the leaching is completed, the zinc sulfate leaching solution is obtained by filtering and separating. The zinc leaching rate is 89.12%. The copper content in the zinc sulfate leaching solution is 0.92g / L, and the iron The content is 0.05g / L. Table 2 shows the contents of zinc sulfide and zinc ferrite in the zinc calcine obtained in the present invention and the traditional zinc smelting zinc calcine.
[0025] Table 2 Zinc sulfide and zinc ferrite content in zinc calcine
[0026] condition
Embodiment 3
[0028] Pyrite and zinc concentrate are mixed evenly in a mass ratio of 2, and pure oxygen is introduced to carry out boiling roasting at 900 °C. After boiling roasting, the temperature is kept at 620 °C for 60 minutes, and the obtained calcined sand is leached at 25 °C. The sulfuric acid content of the leaching solution is 90g / L, the liquid-solid ratio is 10 / 1, and the leaching time is 30min. After the leaching is completed, the zinc sulfate leaching solution is obtained by filtration and separation. The zinc leaching rate is 89.45%, and the copper content in the zinc sulfate leaching solution is 0.95g / L. The content is 0.015g / L.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com