Extraction system for extracting alkaline metals or alkaline-earth metals and application of extraction system
A technology of alkaline earth metals and alkali metals, applied in the field of extraction chemistry and chemical industry, can solve the problems of equipment corrosion, extraction agent emulsification failure, large equipment investment, etc., to reduce equipment corrosion, avoid high acidity, and reduce acid consumption Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
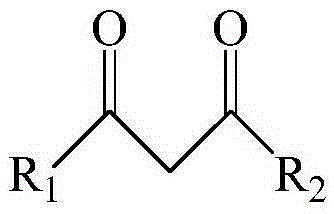
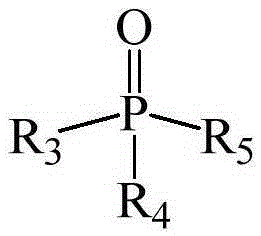
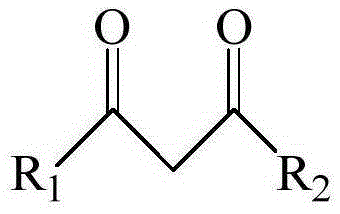
[0051] This example provides three extraction systems, wherein the extractants are dibenzoylmethane, 1-benzoylacetone and 1-(2-naphthoyl)-3,3,3-trifluoroacetone, co-extraction All agents are trioctylphosphine.
[0052] In this embodiment, lithium ions are extracted from the ammonia-ammonium chloride aqueous solution containing lithium ions by using the above three extraction systems; that is, the lithium chloride aqueous solution is used as the extraction water phase. In the extracted aqueous phase, the concentration of lithium ions is 0.02mol / L.
[0053] The specific methods for extracting lithium ions using the above three extraction systems are:
[0054] In step 1, the extraction organic phase is prepared; specifically, the above three extraction systems are respectively mixed with diluent n-dodecane to form the extraction organic phase.
[0055] The concentrations of the above three extractants in their corresponding extracted organic phases are all 0.1mol / L, and the con...
Embodiment 2
[0062] The extraction system used in this example uses 2-thienoyltrifluoroacetone as the extraction agent and trioctylphosphine as the co-extraction agent.
[0063] In this example, the above-mentioned extraction system is used to extract lithium ions from two kinds of ammonia-ammonium chloride aqueous solutions containing lithium ions with different pH values; water box. In the three extraction water phases, the concentration of lithium ions is 0.02 mol / L; the pH values of the two extraction water phases are 12.2 and 13.0 respectively, both of which are weakly alkaline.
[0064] In step one, the extraction organic phase is prepared; specifically, the above extraction system is mixed with the diluent n-dodecane to obtain the extraction organic phase, and in the extraction organic phase, the concentration of the extraction agent 2-thiophenoyltrifluoroacetone is 0.1mol / L, and the concentration of co-extractant trioctylphosphine is 0.2mol / L.
[0065] In step 2, the above-ment...
Embodiment 3
[0071] This example provides three kinds of extraction systems, in which the extractant is benzoyl trifluoroacetone, and the co-extractant is dioctyl methylphosphonate, trioctyl phosphate and tributyl phosphate respectively.
[0072] In this embodiment, the above three extraction systems are used to extract lithium ions from the lithium chloride aqueous solution; that is, the lithium chloride aqueous solution is used as the extraction water phase. In the extracted aqueous phase, the concentration of lithium ions is 0.02mol / L.
[0073] The specific methods for extracting lithium ions using the above three extraction systems are:
[0074] In step 1, the extraction organic phase is prepared; specifically, the above three extraction systems are respectively mixed with diluent n-dodecane to form the extraction organic phase.
[0075] The concentrations of the extractants in their corresponding extracted organic phases are all 0.1mol / L, and the concentrations of the above three co-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com