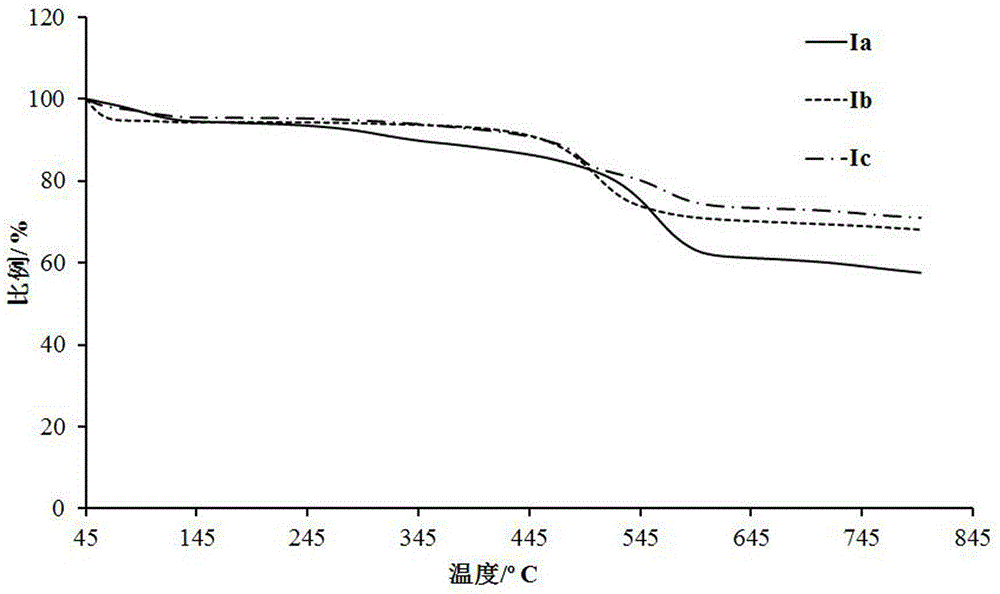
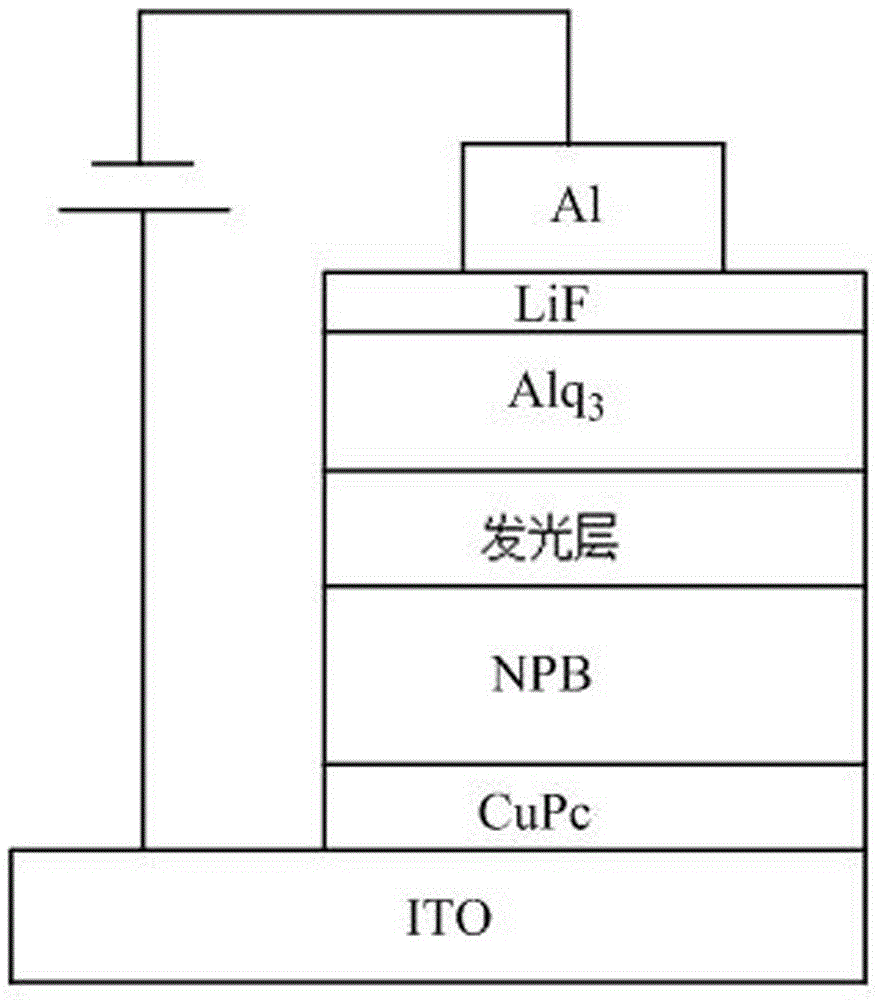
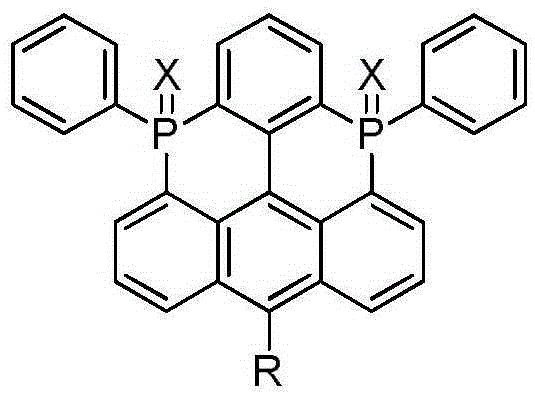
Bisphosphonic heterocyclic organic electroluminescent compounds comprising spirofluorene structures, and synthetic method and application thereof
A spirofluorene structure and compound technology, which is applied in the field of organic electroluminescent materials to achieve the effects of good thermal stability, reduced LUMO orbital, and improved electron injection and transport capabilities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] The synthesis of embodiment 1 compound Ia
[0029]
[0030] Synthesis of compound Ⅳ
[0031] Under argon protection, 9-anthraceneboronic acid (2.2g), 2,5-dibromoiodobenzene (3.6g), tetrahydrofuran (30mL), water (30mL), tetrakis(triphenylphosphine) were added successively to the flask Palladium (100mg), potassium carbonate (2.7g), heated under reflux for 10h. Stop the reaction, extract three times with ethyl acetate, combine the organic phases, and then wash to neutrality; separate the organic phases, add anhydrous magnesium sulfate to dry, suction filter, and spin dry; silica gel column chromatography gives 3.6 g of white solids, the yield 88%.
[0032] Synthesis of Compound V
[0033] Under argon protection, compound IV (3.0 g) and tetrahydrofuran (50 mL) were added to the flask, then the system was cooled to -78 ° C, and a 2.5 M n-hexane solution containing n-butyllithium (6 mL) was added dropwise to the system. ), react at -78°C for 1 hour after the dropwise a...
Embodiment 2
[0042] The synthesis of embodiment 2 compound 1b
[0043]
[0044] Synthesis of compound Ⅵb
[0045] Compound V (3.0 g) obtained according to the synthesis method of Example 1, toluene (20 mL), and 10% palladium carbon (100 mg) were added to the flask, and the reaction was heated under reflux for 10 hours. After returning to room temperature, excess sulfur powder was added to the system, reacted for 5 hours, and spin-dried; silica gel column chromatography yielded 2.0 g of a light yellow solid, with a yield of 78%.
[0046] Synthesis of Compound Ⅶb
[0047] Add compound VIb (2.0 g), glacial acetic acid (20 mL), and liquid bromine (2 g) into the flask, and heat to reflux for 5 hours. Stop the reaction, add saturated sodium thiosulfate solution (20 mL), extract three times with ethyl acetate, combine the organic phases, remove the solvent, add ethyl acetate (10 mL) again to dissolve, dry over anhydrous magnesium sulfate, filter with suction, and spin dry; Silica gel column c...
Embodiment 3
[0052] The synthesis of embodiment 3 compound Ic
[0053]
[0054] Synthesis of Compound Ⅵc
[0055] Compound V (3.0 g) obtained according to the synthesis method of Example 1, toluene (20 mL), and 10% palladium carbon (100 mg) were added to the flask, and the reaction was heated under reflux for 10 hours. After returning to room temperature, excess selenium powder was added to the system, reacted for 5 hours, and spin-dried; silica gel column chromatography yielded 2.3 g of a light yellow solid, with a yield of 76%.
[0056] Synthesis of Compound Ⅶc
[0057] Add compound VIc (2.0 g), glacial acetic acid (20 mL), and liquid bromine (2 g) into the flask, and heat to reflux for 7 hours. Stop the reaction, add saturated sodium thiosulfate solution (20 mL), extract three times with ethyl acetate, combine the organic phases, remove the solvent, add ethyl acetate (10 mL) again to dissolve, dry over anhydrous magnesium sulfate, filter with suction, and spin dry; Silica gel colu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com