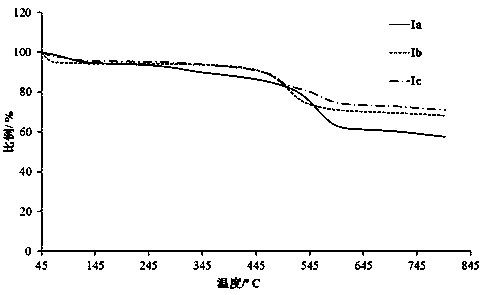
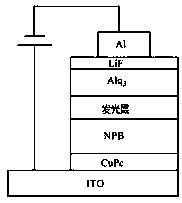
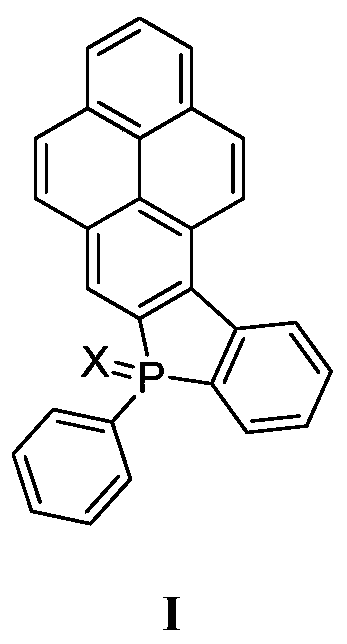
A kind of phosphine heterocyclic compound, synthesis method and application thereof
A compound and ring technology, applied in the field of organic electroluminescent materials, can solve the problems of low efficiency of OLED devices, unbalanced carrier transport, low thermal stability, etc., achieve good thermal stability, improve efficiency, and reduce LUMO track effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] The synthesis of embodiment 1 compound Ia
[0025]
[0026] Synthesis of compound Ⅱ
[0027] Under argon protection, 1-pyreneboronic acid (24.6g), o-bromoiodobenzene (28.2g), toluene (300mL), water (30mL), and tetrakis(triphenylphosphine) palladium (200mg) were successively added to the flask. , Potassium carbonate (27.6g), heated to reflux for 10h. Stop the reaction, extract three times with ethyl acetate, combine the organic phases, and wash with water until neutral; separate the organic phases, add anhydrous magnesium sulfate to dry, filter with suction, and spin dry; silica gel column chromatography yields 30.3 g of compound II as a white solid, Yield 85%.
[0028] Synthesis of compound Ⅲ
[0029] Under the protection of argon, compound II (20g) and tetrahydrofuran (200mL) were added to the flask, then the system was cooled to -78°C, and a 2.5M n-hexane solution (25mL) containing n-butyllithium was added dropwise to the system , after the dropwise addition, r...
Embodiment 2
[0033] ESI, m / z: [M+H] + calcd for C 28 h 18 OP, theoretical value: 401.1095; measured value: 401.1093. The synthesis of embodiment 2 compound 1b
[0034]
[0035] Compound III (5.0 g), toluene (100 mL) and palladium acetate (120 mg) obtained according to the synthesis method of Example 1 were added into the flask, and the reaction was heated under reflux for 10 hours. After returning to room temperature, excess sulfur powder was added to the system, reacted for 5 hours, and spin-dried; silica gel column chromatography yielded 3.4 g of a light yellow solid, with a yield of 76%.
[0036] 31 PNMR (162Hz, CDCl 3 ), δ: 34; 1 HNMR (400MHz, CDCl 3 ), δ:6.71~6.89(m,5H),7.04~7.72(m,10H),7.83~8.05(m,2H);
[0037] ESI, m / z: [M+H] + calcd for C 28 h 18 PS, theoretical value: 417.0867; measured value: 417.0864. The synthesis of embodiment 3 compound Ic
Embodiment 3
[0037] ESI, m / z: [M+H] + calcd for C 28 h 18 PS, theoretical value: 417.0867; measured value: 417.0864. The synthesis of embodiment 3 compound Ic
[0038]
[0039] Compound III (5.0 g), toluene (100 mL) and palladium acetate (120 mg) obtained according to the synthesis method of Example 1 were added into the flask, and the reaction was heated under reflux for 10 hours. After returning to room temperature, excess selenium powder was added to the system, reacted for 5 hours, and spin-dried; silica gel column chromatography gave 3.8 g of a light yellow solid, with a yield of 75%.
[0040] 31 PNMR (162Hz, CDCl 3 ), δ: 37; 1 HNMR (400MHz, CDCl 3 ), δ:6.70~6.89(m,5H),7.03~7.74(m,10H),7.81~8.03(m,2H);
[0041] ESI, m / z: [M+H] + calcd for C 28 h 18 SeP, theoretical: 465.0311; found: 465.0310.
[0042] The following is the application of the compounds of the present invention
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com