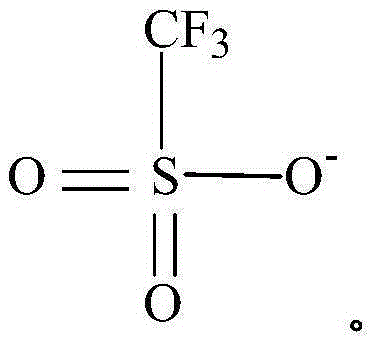
Racemo selective metallation process
A racemization and metallization technology, applied in organic chemistry methods, chemical instruments and methods, metallocenes, etc., can solve problems such as inconsistent effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0285] E1. A method comprising:
[0286] Contacting the deprotonated bridged bis(indenyl) compound with a racemic directing metallation reagent to form a product mixture enriched in the bridged bis(indenyl) relative to any formed meso isomer base) racemic isomers of metallocene transition metal compounds,
[0287] wherein said racemic-directing metallation reagent is represented by the following formula:
[0288] m d x e Y f Z g (preferably M * x 2 Y 2 Z 2 , where M * for Group 4 metals),
[0289] Wherein M, X, Y and Z are defined as follows, d is the coordination number of metal M and is 4, 5 or 6, e is 2, 3, 4 or 5, f is 1, 2, 3 or 4 and g is 0, 1 or 2, where e+f=d;
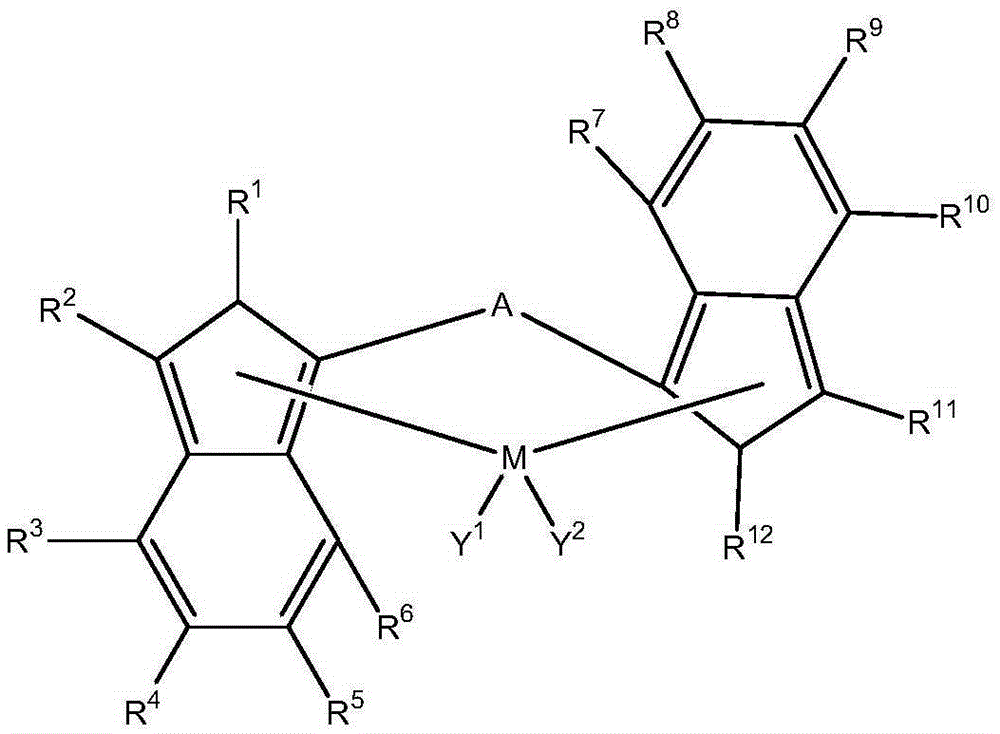
[0290] Wherein the bis(indenyl) metallocene transition metal compound is represented by the following formula:
[0291]
[0292] wherein M is a metal of group 4, 5 or 6 of the periodic table;
[0293] Where A is a divalent group, including C 1 -C 20 A hydrocarbyl group, a functional group com...
Embodiment 1
[0379] The deprotonated bridged bis(4- (o-tolyl)-2-methylindenyl) compound. At this point, the reaction mixture was cooled to -75°C. The deprotonated bridged bis(4-(o-tolyl)-2-methylindenyl) compound was then mixed with a stoichiometrically equivalent amount of ZrCl 2 (O-t-Bu) 2 (thf) 2 Contact (see Njua, E.Y. et al., ZrCl in Inorg.Chem., 2010,49,2163 2 (O-t-Bu) 2 (thf) 2 The preparation method of), ZrCl 2 (O-t-Bu) 2 (thf) 2 Introduced into the reaction mixture as a solid. The product mixture was warmed to room temperature (25°C) while stirring overnight. After concentration, filtration and drying, the crude product was obtained. The crude product was slurried in ether and the bridged bis(4-(o-tolyl)-2-methylindenyl)metallocene product was isolated as a yellow solid after filtration. The product, dimethylsilylbis[4-(o-methylphenyl)-2-methylindenyl]Zr(O-t-Bu) 2 , as the pure racemic isomer; by 1 HNMR did not observe the meso isomer.
Embodiment 2
[0383] Dimethylsilylbis[4-(phenyl)-2-methylindenyl]Zr(O-t-Bu) 2 The catalyst precursor is used to polymerize ethylene and hexene. This precursor was prepared according to the general procedure in Example 1 and approximately 3:1 rac, meso isomers were obtained. Afterwards, the precursor was purified by washing with ether and then recrystallized from a dichloromethane / pentane solvent mixture.
[0384] A 30 wt% solution of MAO in toluene (Albemarle; 0.7951 g; 4.112 mmol) was dissolved in 1.5 mL of toluene; the solution was stirred for 15 minutes. To this solution was added the catalyst precursor (29.9 mg; 0.0415 mmol) as a solid, and the residue was washed with 0.5 mL of toluene. The reaction mixture was stirred for 20 minutes. To the reaction mixture was added 1.0404 g of Silica-948 (Grace), which had been previously calcined at 600°C. The wet mixture was stirred with a spatula for 10 minutes, then dried under vacuum. 15 mL of hexene and 0.1 mL of tris(n-octyl)aluminum were...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com