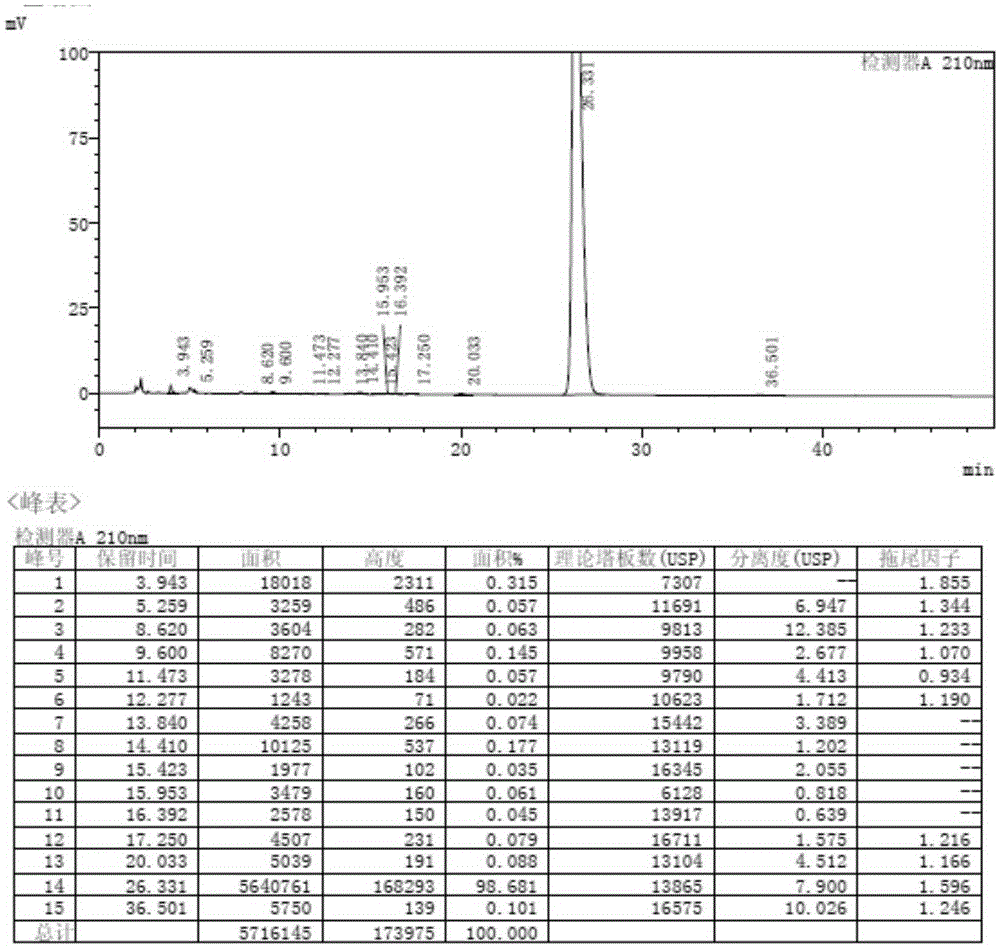
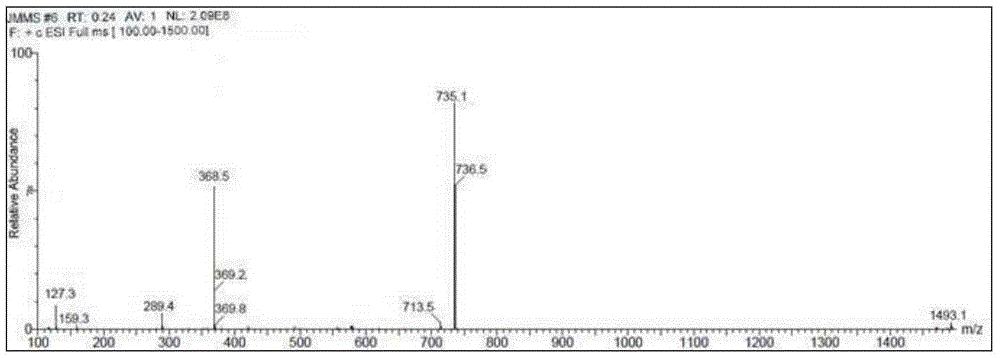
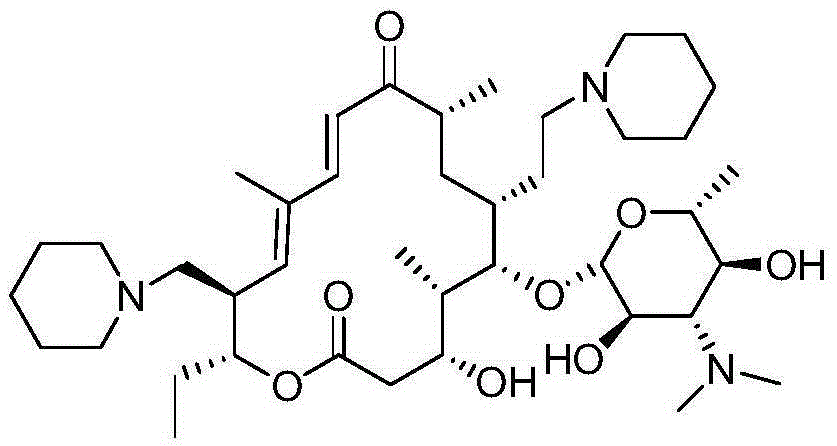
Tildipirosin preparation method
A technology of Tedirosin and Tylenolactone, which is applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve problems such as strict operating conditions, reduce operating steps, reduce steps, and reduce costs Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] (1) Preparation of 23-hydroxy-5-0-mycaminosyl-tylonolide (Ⅱ)
[0030] Add 30g (32.7mml) tylomycin to 300ml water, stir and heat up to 35-40 degrees, slowly add 27.6g48% HBr (163.5mml), keep warm for 2h, detect that the raw material content is less than 2%, add 16.6 g48% HBr (98.1mml), warm up to 45-50 degrees, continue to stir for 5 hours, cool down to room temperature, extract twice with 180ml ethyl acetate, cool the water phase to 4-8 degrees, and use 5mol / L NaOH Adjust the pH of the solution to about 10, extract three times with 180ml of dichloromethane, combine the organic phases, dry, concentrate under reduced pressure, beat with 60ml of methyl tert-butyl ether, filter, and dry to obtain 14.1g of 23-hydroxy-5-0 -mycaminosyl-tylonolide, yield 72.3%.
[0031] (2) Preparation of 14-formyl-5-0-mycaminosyl-tylonolide (Ⅲ)
[0032] The 23-hydroxyl-5-O-mycaminosyl-tylonolide (58.6mml) of 35g, 12.1gNaNO2 (175.8mml), 3.0g acetic anhydride (29.3mmol) were added in the aceti...
Embodiment 2
[0036] (1) Preparation of 23-hydroxy-5-0-mycaminosyl-tylonolide (Ⅱ)
[0037] Add 30g (32.7mml) tylomycin to 300ml water, stir and heat up to 50-55 degrees, slowly add 22.1g48% HBr (130.8mml), keep warm for 2h, detect that the raw material content is less than 2%, add 22.1 g48% HBr (130.8mml), heat up to 50-55 degrees, continue to stir for 5 hours, cool down to room temperature, extract twice with 180ml ethyl acetate, cool the water phase to 4-8 degrees, and use 5mol / L NaOH Adjust the pH of the solution to about 10, extract three times with 180ml of dichloromethane, combine the organic phases, dry, concentrate under reduced pressure, beat with 60ml of methyl tert-butyl ether, filter, and dry to obtain 13.1g of 23-hydroxy-5-0 -mycaminosyl-tylonolide, yield 67.0%.
[0038] (2) Preparation of 14-formyl-5-0-mycaminosyl-tylonolide (Ⅲ)
[0039]The 23-hydroxyl-5-O-mycaminosyl-tylonolide (58.6mml) of 35g, 20.2gNaNO2 (293.0mml) 6.0g acetic anhydride (58.6mmol) are added in the 50ml ac...
Embodiment 3
[0043] (1) Preparation of 23-hydroxy-5-0-mycaminosyl-tylonolide (Ⅱ)
[0044] Add 30g (32.7mml) tylomycin to 300ml water, stir and heat up to 35-40 degrees, slowly add 44.1g48% HBr (261.6mml), keep warm for 2 hours, when the content of raw materials is detected to be less than 2%, add 16.6 g48% HBr (65.4mml), warm up to 40-45 degrees, continue to stir for 5h, cool down to room temperature, extract twice with 180ml ethyl acetate, cool the water phase to 4-8 degrees, and use 5mol / L NaOH Adjust the pH of the solution to about 10, extract three times with 180ml of dichloromethane, combine the organic phases, dry, concentrate under reduced pressure, beat with 60ml of methyl tert-butyl ether, filter, and dry to obtain 13.5g of 23-hydroxy-5-0 -mycaminosyl-tylonolide, yield 69.1%.
[0045] (2) Preparation of 14-formyl-5-0-mycaminosyl-tylonolide (Ⅲ)
[0046] 35g of 23-hydroxy-5-O-mycaminosyl-tylonolide (58.6mml), NaNO2 (1:2-5), acetic anhydride (1:0.2-1) were added to 30ml of acetic a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com