Use of medicine composition in preparation of medicines treating qi-stagnation blood-stasis type chloasma
A technology of qi stagnation and blood stasis type and composition, which is applied in the field of preparing medicines for treating chloasma of qi stagnation and blood stasis type. The effect of drug stability of the preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
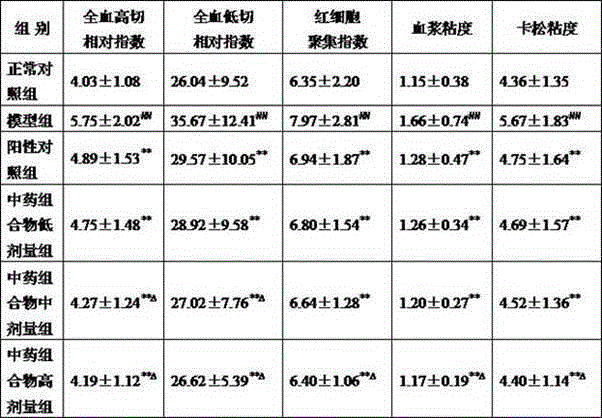
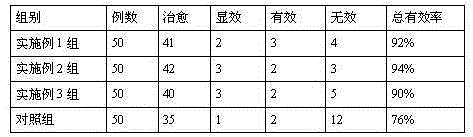
[0034] The pharmaceutical composition is prepared from the following raw materials in parts by weight: 10 parts of Moringa leaves, 9 parts of black trigonum, 8 parts of motherwort, 8 parts of curcuma, 6 parts of lily, 6 parts of scallion, 5 parts of hawthorn, bergamot 5 parts, 3 parts of fresh rehmannia, 3 parts of mulberry, 3 parts of jujube, 3 parts of ginseng and 2 parts of licorice.
[0035] The preparation method is as follows:
[0036] S1: Take leaves of Moringa oleifera oleifera, wash, dry, crush, pass through a 150-mesh sieve, add 10 times the amount of ethanol with a volume fraction of 75% of the total amount of medicinal materials, and then add 2% of the total amount of medicinal materials Helicase, soak at 38°C Extract for 1 hour, then reflux extraction at 85°C for 5 hours, filter, and concentrate the filtrate under reduced pressure to an extract with a relative density of 1.20 at 60°C to obtain Extract A;
[0037]S2: Take black trigonum, motherwort, turmeric, lily...
Embodiment 2
[0041] The pharmaceutical composition is prepared from the following preparation raw materials in parts by weight: 15 parts of Moringa leaves, 12 parts of black trigonum, 10 parts of motherwort, 10 parts of curcuma, 8 parts of lily, 8 parts of scallion, 7 parts of hawthorn, bergamot 7 parts, 6 parts of fresh rehmannia, 6 parts of mulberry, 6 parts of jujube, 6 parts of ginseng and 5 parts of licorice.
[0042] The preparation method is as in Example 1.
Embodiment 3
[0044] The pharmaceutical composition is prepared from the following raw materials in parts by weight: 25 parts of Moringa leaves, 20 parts of black trigonum, 15 parts of motherwort, 12 parts of curcuma, 10 parts of lily, 10 parts of scallion, 10 parts of hawthorn, bergamot 10 parts, 9 parts of fresh rehmannia, 9 parts of mulberry, 9 parts of jujube, 9 parts of ginseng and 10 parts of licorice.
[0045] The preparation method is as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com