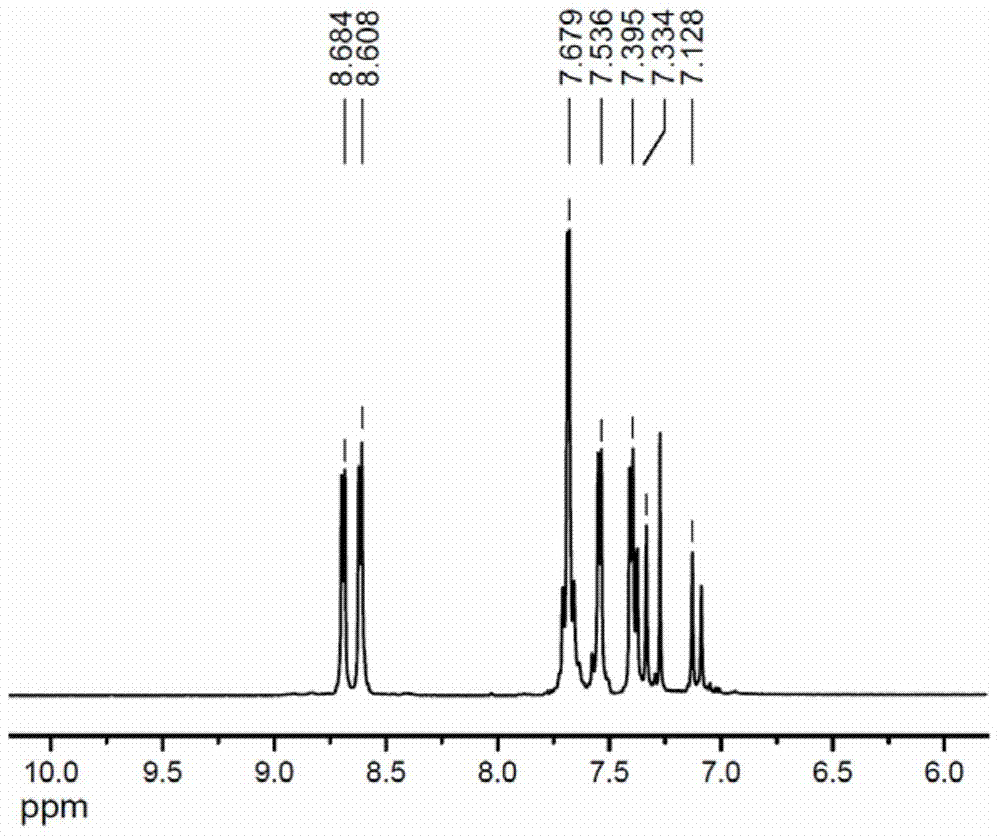
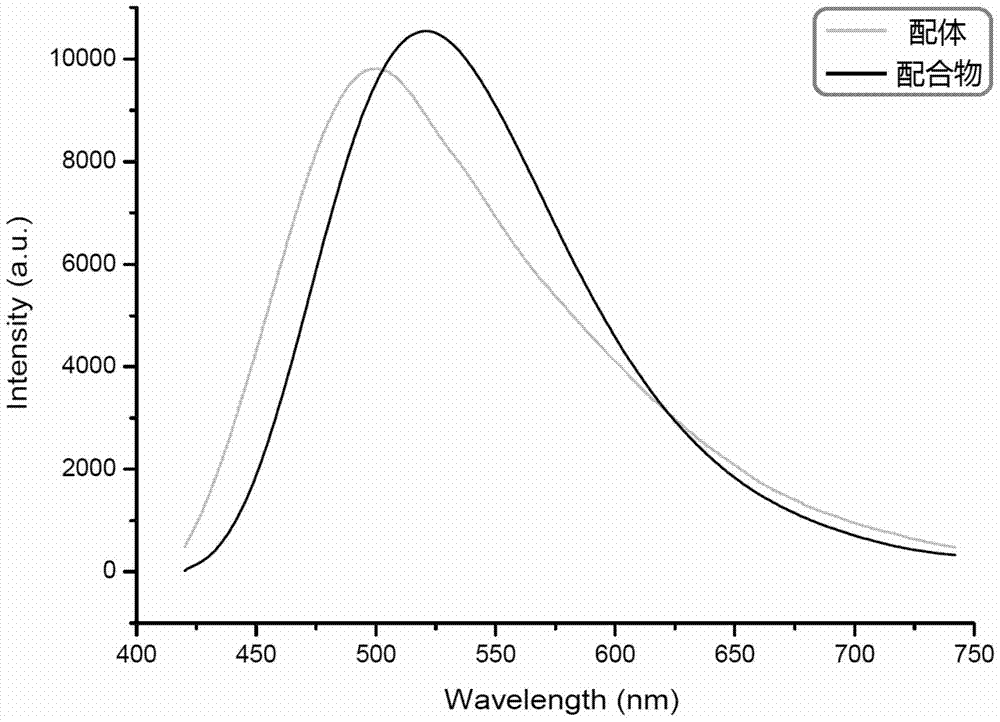
A kind of 1-(4-pyridyl)-4-(4'-pyridylvinyl)benzene and its preparation method and application
A pyridyl vinyl, pyridyl technology, applied in the direction of zinc organic compounds, organic chemistry, etc., can solve the problem that bipyridine ligands are rarely studied.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0019] Non-limiting examples are described as follows:
[0020] p-Bromoiodobenzene, 4-vinylpyridine, palladium chloride, hydrazine hydrate, 4-pyridineboronic acid, cesium carbonate, and tetrakis(triphenylphosphine)palladium were all purchased from Shanghai Bailingwei Chemical Technology Co., Ltd., and 200-300 mesh silica gel was purchased from Purchased from Qingdao Ocean Chemical Plant, ethyl acetate, petroleum ether, N,N′-dimethylformamide and triethylamine were purchased from Sinopharm Chemical Reagent Co., Ltd.
[0021] 1. Preparation of divalent palladium catalyst
[0022] 0.18 g (100 mmol) of palladium chloride and 5 mL of 50% hydrazine hydrate were mixed and stirred for about 2 hours, filtered to obtain a gray solid, which was sealed and stored after vacuum drying.
[0023] 2. Preparation of Intermediates
[0024] Dissolve 5.66g (20mmol) of p-bromoiodobenzene in 25mL of N,N'-dimethylformamide, then add 35mg of divalent palladium catalyst, 2.52g (24mmol) of 4-vinylpyri...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com