Cyclo-alpha-enaminone compound, synthetic method and application thereof, and tobacco product
The technology of a cyclomethenamine and a synthesis method, which is applied in the field of heterocyclic compounds and cooling agents, can solve the problems of unpleasant smell amine substances, low cooling effect, poor stability, etc., and achieve obvious cooling taste and improved Quality, good stability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
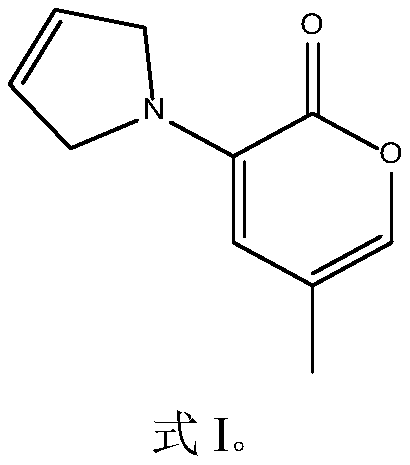
[0034] The cyclomethanaminoketone compound of this embodiment has the structure shown in formula I, and is named 3-(3-pyrrolinyl)-5-methyl-2H-pyran-2-one:
[0035]
[0036] The embodiment of the synthetic method of cyclomethanyl ketone compound
Embodiment 2
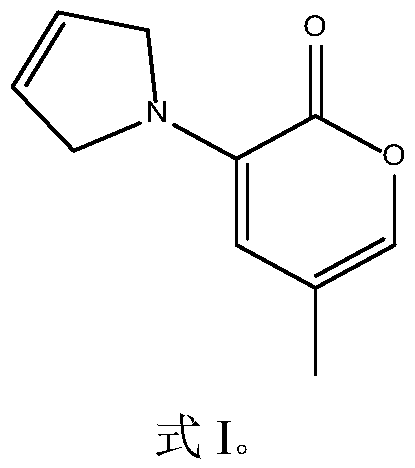
[0038] The present embodiment has the synthetic route of the cyclic methyl enamino ketone compound of the structure shown in formula I as follows:
[0039]
[0040] The specific synthetic method comprises the following steps:
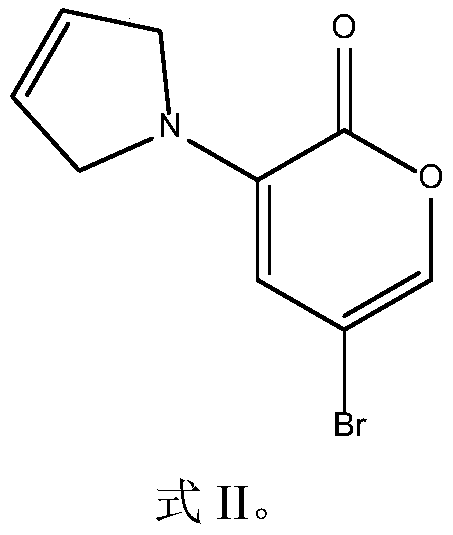
[0041] 1) Synthesis of 3-(3-pyrrolinyl)-5-bromo-2H-pyran-2-one (the compound represented by formula II)
[0042] Under nitrogen protection, 254 mg (1 mmol) of 3,5-dibromo-2-pyrone, 7 mg (0.03 mmol) of palladium acetate, and 1,1'-binaphthalene-2,2' were successively added to a dry Schlenk reaction tube. - 37 mg (0.06 mmol) of bisdiphenylphosphine, 207 mg (1.5 mmol) of potassium carbonate, 83 mg (1.2 mmol) of 3-pyrroline, and 3 mL of anhydrous toluene, the reaction solution was stirred at 80°C for 1 hour under nitrogen atmosphere. The reaction solution was cooled to room temperature, filtered through a short silica gel column, the eluent was 40 mL of diethyl ether, and the filtrate was evaporated under reduced pressure to remove the solvent to obtain ...
Embodiment 3
[0048] The application of the cyclomethanamine compound as a cooling agent in this embodiment is specifically the application of the cyclomethanaminone compound having the structure shown in formula I as a cooling agent in cigarettes.
[0049] In another embodiment of the application of the cyclomethicone compound of the present invention as a cooling agent, it is the application of the compound of formula I as a cooling agent in chewing gum, beverage, toothpaste or cosmetics.
[0050] Examples of Tobacco Products
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com