Pentacyclic triterpene compound and application thereof
A technology for pentacyclic triterpenoids and compounds is applied in the field of pentacyclic triterpenoids, which can solve the problems of insufficient research on chemical constituents, and achieve the effects of good anti-inflammatory activity and good safety.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0027] The technical solutions of the present invention will be further described below in conjunction with the embodiments.
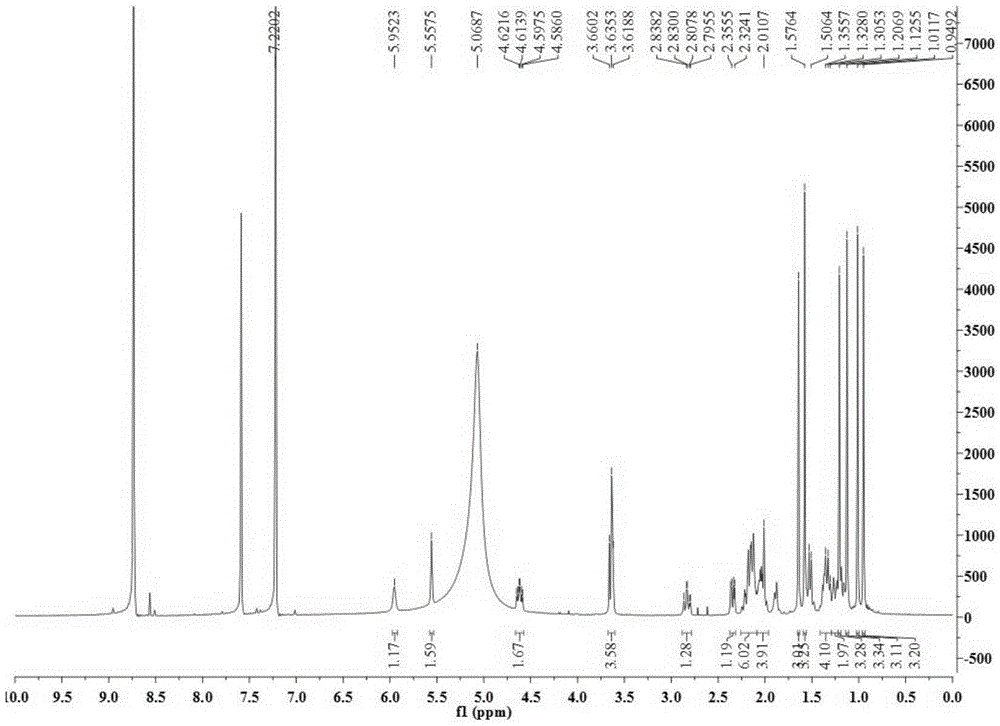
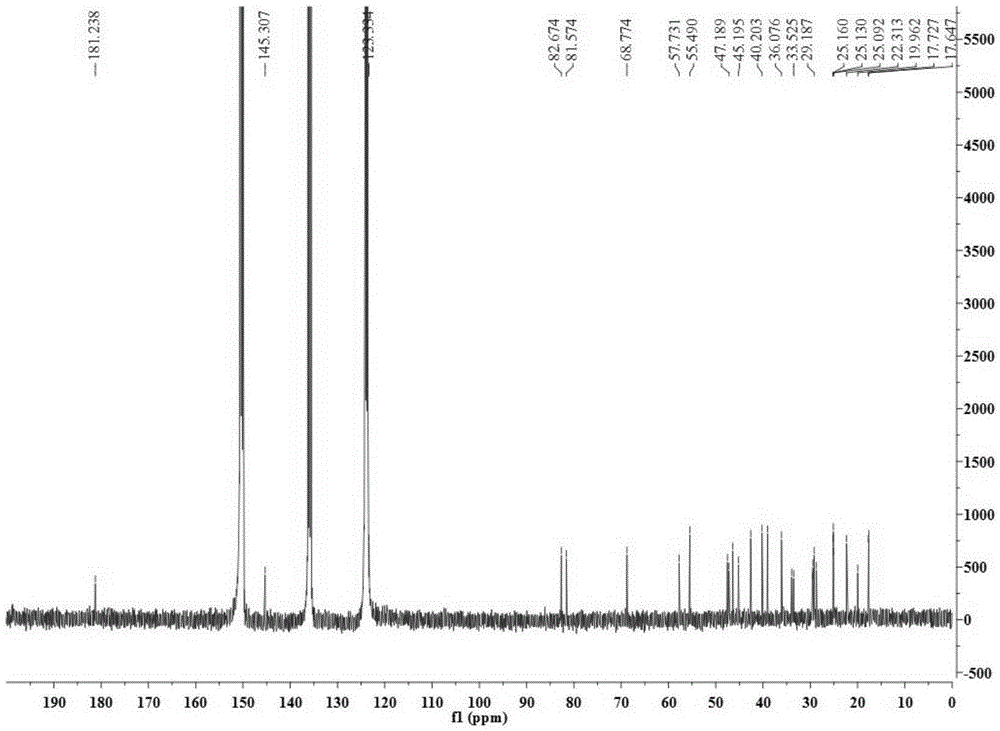
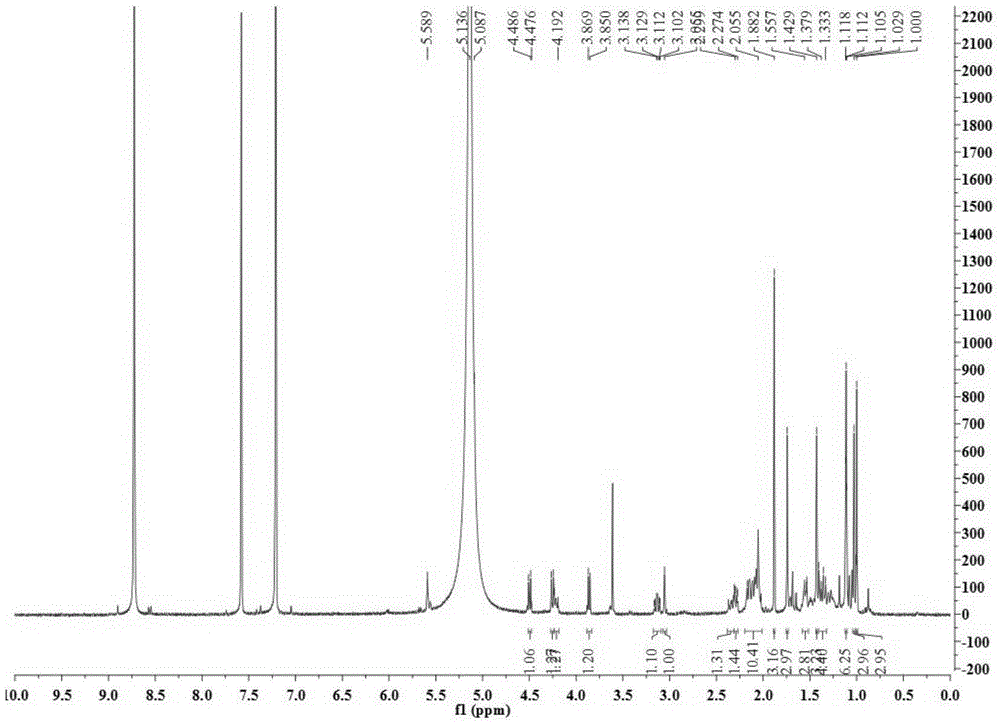
[0028] Take 34.5Kg of Quercus brevifolia seeds, heat and reflux extraction with 70% ethanol for 4 times, each time for 4 hours, combine the extracts and recover the solvent under reduced pressure to obtain a total concentrated solution of 30L, and use cyclohexane, ethyl acetate and n-butanol successively Equal volumes were extracted 4 times each, and 200 g of the ethyl acetate layer extract was obtained. Compound 1-4 of the present invention was obtained by separating from the ethyl acetate layer extract by using separation methods such as silica gel column chromatography, SephdaxLH-20 gel chromatography, ODS medium and low pressure column chromatography, and reverse-phase high-performance liquid chromatography. The structures of the above four compounds were identified by physical and chemical constants and modern spectroscopy methods (HRESIMS, 1D-NMR...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com